Pembrolizumab Plus Carboplatin and Paclitaxel as First-Line Therapy for Recurrent/Metastatic Head and Neck Squamous Cell Carcinoma (KEYNOTE-B10): A Single-Arm Phase IV Trial
- PMID: 39038265
- PMCID: PMC11361359
- DOI: 10.1200/JCO.23.02625
Pembrolizumab Plus Carboplatin and Paclitaxel as First-Line Therapy for Recurrent/Metastatic Head and Neck Squamous Cell Carcinoma (KEYNOTE-B10): A Single-Arm Phase IV Trial
Abstract
Purpose: Standard-of-care first-line treatment for recurrent/metastatic head and neck squamous cell carcinoma (R/M HNSCC) is pembrolizumab plus platinum and fluorouracil (FU). However, FU is associated with potential challenges (continuous 4-day infusion, high administration costs, and cardiovascular and gastrointestinal toxicities), creating a clinical need for alternative chemotherapy combinations. We evaluated the efficacy and safety of first-line pembrolizumab plus carboplatin and paclitaxel for R/M HNSCC in the open-label, single-arm, phase IV KEYNOTE-B10 study (ClinicalTrials.gov identifier: NCT04489888).
Methods: Eligible adults had previously untreated, histologically or cytologically confirmed R/M HNSCC regardless of PD-L1 status, measurable disease per RECIST v1.1 by blinded independent central review (BICR), and an Eastern Cooperative Oncology Group performance status of 0 or 1. Patients received pembrolizumab 200 mg intravenously once every 3 weeks for ≤35 cycles and carboplatin AUC 5 mg/mL/min intravenously once every 3 weeks for ≤6 cycles and investigator's choice of paclitaxel 100 mg/m2 on days 1 and 8 or 175 mg/m2 on day 1, intravenously once every 3 weeks. The primary end point was objective response rate per RECIST v1.1 by BICR.
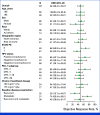
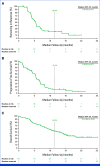
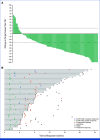
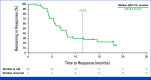
Results: Between October 27, 2020, and April 29, 2022, 149 patients were screened and 101 received treatment. As of February 20, 2023, the median follow-up was 18.9 months (range, 9.1-27.0). At this final analysis, 49 (49%) of 101 patients had an objective response (95% CI, 38.4 to 58.7), including seven patients (7%) with a confirmed complete response. Of the 101 treated patients, grade 3-5 and serious treatment-related adverse events occurred in 76 (75%) and 27 (27%), respectively. There were no new safety signals.
Conclusion: Pembrolizumab plus carboplatin and paclitaxel showed promising antitumor activity and a manageable safety profile in first-line R/M HNSCC, suggesting this combination may be an alternative option for this patient population.
Conflict of interest statement
The following represents disclosure information provided by authors of this manuscript. All relationships are considered compensated unless otherwise noted. Relationships are self-held unless noted. I = Immediate Family Member, Inst = My Institution. Relationships may not relate to the subject matter of this manuscript. For more information about ASCO's conflict of interest policy, please refer to
Open Payments is a public database containing information reported by companies about payments made to US-licensed physicians (
No other potential conflicts of interest were reported.
Figures

Comment in
-
First-line therapy for recurrent metastatic head and neck squamous cell carcinoma: every rose has its thorn.Transl Cancer Res. 2025 Jul 30;14(7):3887-3891. doi: 10.21037/tcr-2025-782. Epub 2025 Jul 25. Transl Cancer Res. 2025. PMID: 40792138 Free PMC article. No abstract available.
References
-
- Burtness B, Harrington KJ, Greil R, et al. : Pembrolizumab alone or with chemotherapy versus cetuximab with chemotherapy for recurrent or metastatic squamous cell carcinoma of the head and neck (KEYNOTE-048): A randomised, open-label, phase 3 study. Lancet 394:1915-1928, 2019 - PubMed
-
- Merck : KEYTRUDA (pembrolizumab) injection, for intravenous use [prescribing information]. https://www.merck.com/product/usa/pi_circulars/k/keytruda/keytruda_pi.pdf
-
- Guigay J: Are there alternative chemotherapy regimens for EXTREME in recurrent/metastatic squamous cell carcinoma of the head and neck (R/M-SCCHN)?, in Vermorken JB, Budach V, Leemans CR, et al. (eds): Critical Issues in Head and Neck Oncology: Key Concepts from the Sixth THNO Meeting. Cham, Switzerland, Springer International Publishing, 2018, pp 267-276
-
- Vermorken JB, Mesia R, Rivera F, et al. : Platinum-based chemotherapy plus cetuximab in head and neck cancer. N Engl J Med 359:1116-1127, 2008 - PubMed
Publication types
MeSH terms
Substances
Associated data
Grants and funding
LinkOut - more resources
Full Text Sources
Medical
Research Materials
Miscellaneous

