Curative Strategy for High-Risk Smoldering Myeloma: Carfilzomib, Lenalidomide, and Dexamethasone (KRd) Followed by Transplant, KRd Consolidation, and Rd Maintenance
- PMID: 39038268
- PMCID: PMC11404760
- DOI: 10.1200/JCO.23.02771
Curative Strategy for High-Risk Smoldering Myeloma: Carfilzomib, Lenalidomide, and Dexamethasone (KRd) Followed by Transplant, KRd Consolidation, and Rd Maintenance
Abstract
Purpose: Early treatment of high-risk smoldering myeloma has been shown to delay progression to multiple myeloma (MM). We conducted this trial with curative intention using a treatment approach employed for newly diagnosed patients with MM.
Methods: Patients with high-risk smoldering myeloma (>50% progression risk at 2 years) and transplant candidates were included and received induction therapy with carfilzomib, lenalidomide, and dexamethasone (KRd), six cycles, followed by high-dose melphalan (200 mg/m2) autologous stem-cell transplantation (HDM-ASCT), two KRd consolidation cycles, and Rd maintenance for 2 years. The primary end point was undetectable measurable residual disease (uMRD) rate by next-generation flow after ASCT. Sustained uMRD 4 years after ASCT was the secondary end point.
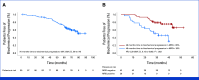
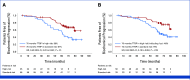
Results: Between June 2015 and June 2017, 90 patients were included, and 31% met at least one SixtyLightchain MRI (SLiM)-hypercalcemia, renal impairment, anemia, bone disease (CRAB) criterion. After a median follow-up of 70.1 months, 3 months after ASCT, in the intention-to-treat population, 56 (62%) of 90 patients had uMRD, and 4 years later, it was sustained in 29 patients (31%). Five patients progressed to MM, and the 70-month progression rate was 94% (95% CI, 84 to 89). The presence of any SLiM CRAB criteria predicted progression to MM (four of the five patients; hazard ratio, 0.12; 95% CI, 0.14 to 1.13; P = .03). Thirty-six patients showed biochemical progression, and failure to achieve uMRD at the end of treatment predicted it. The 70-month overall survival was 92% (95% CI, 82 to 89). Neutropenia and infections were the most frequent adverse events during treatment, resulting in one treatment-related death. Three second primary malignancies have been reported.
Conclusion: Although a longer follow-up is needed, this curative approach is encouraging and more effective than active MM, with 31% of the patients maintaining the uMRD 4 years after HDM-ASCT.
Trial registration: ClinicalTrials.gov NCT02415413.
Conflict of interest statement
The following represents disclosure information provided by authors of this manuscript. All relationships are considered compensated unless otherwise noted. Relationships are self-held unless noted. I = Immediate Family Member, Inst = My Institution. Relationships may not relate to the subject matter of this manuscript. For more information about ASCO's conflict of interest policy, please refer to
Open Payments is a public database containing information reported by companies about payments made to US-licensed physicians (
Figures


References
-
- Kyle RA, Remstein ED, Therneau TM, et al. : Clinical course and prognosis of smoldering (asymptomatic) multiple myeloma. N Engl J Med 356:2582-2590, 2007 - PubMed
-
- Pérez-Persona E, Vidriales MB, Mateo G, et al. : New criteria to identify risk of progression in monoclonal gammopathy of uncertain significance and smoldering multiple myeloma based on multiparameter flow cytometry analysis of bone marrow plasma cells. Blood 110:2586-2592, 2007 - PubMed
-
- Mateos MV, Hernández MT, Salvador C, et al. : Lenalidomide-dexamethasone versus observation in high-risk smoldering myeloma after 12 years of median follow-up time: A randomized, open-label study. Eur J Cancer 174:243-250, 2022 - PubMed
-
- Mateos MV, Hernández MT, Giraldo P, et al. : Lenalidomide plus dexamethasone for high-risk smoldering multiple myeloma. N Engl J Med 369:438-447, 2013 - PubMed
MeSH terms
Substances
Associated data
LinkOut - more resources
Full Text Sources
Medical
Research Materials
Miscellaneous

