Cervical spinal cord morphometrics in degenerative cervical myelopathy: quantification using semi-automated normalized technique and correlation with neurological dysfunctions
- PMID: 39038658
- PMCID: PMC11527586
- DOI: 10.1016/j.spinee.2024.07.002
Cervical spinal cord morphometrics in degenerative cervical myelopathy: quantification using semi-automated normalized technique and correlation with neurological dysfunctions
Abstract
Background context: Degenerative cervical myelopathy (DCM) is characterized by spinal cord atrophy. Accurate estimation of spinal cord atrophy is key to the understanding of neurological diseases, including DCM. However, its clinical application is hampered by difficulties in its precise and consistent estimation due to significant variability in spinal cord morphometry along the cervical spine, both within and between individuals.
Purpose: To characterize morphometrics of the compressed spinal cord in DCM patients. We employed our semiautomated analysis framework that incorporates the Spinal Cord Toolbox (SCT) and a normalization approach to effectively address the challenges posed by cord compression in these patients. Additionally, we examined the clinical relevance of these morphometric measures to enhance our understanding of DCM pathophysiology.
Study design: Prospective study.
Patient sample: This study investigated 36 DCM patients and 31 healthy controls (HCs).
Outcome measures: Clinical scores including 9-hole peg test for hand dexterity, hand grip strength, balance, gait speed, modified Japanese Orthopaedic Association (mJOA) score, and imaging-based spinal cord morphometrics.
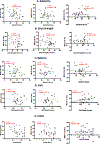
Method: Using the generic spine acquisition protocol and our semiautomated analysis pipeline, spinal cord morphometrics, including cross-sectional area (CSA), anterior-posterior (AP) and transverse (RL) diameters, eccentricity, and solidity, were estimated from sagittal T2w magnetic resonance imaging (MRI) images using the Spinal Cord Toolbox (SCT). Normalized metrics were extracted from the C1 to C7 vertebral levels and compared between DCM patients and HC. Morphometric data at regions of maximum spinal cord compression (MSCC) were correlated with the clinical scores. A subset of participants underwent follow-up scans at 6 months to monitor longitudinal changes in spinal cord atrophy.
Results: Spinal cord morphometric data were normalized against the healthy population morphometry (PAM50 database) and extracted for all participants. DCM patients showed a notable reduction in CSA, AP, and RL diameter across all vertebral levels compared to HC. MSCC metrics correlated significantly with clinical scores like dexterity, grip strength, and mJOA scores. Longitudinal analysis indicated a decrease in CSA and worsening clinical scores in DCM patients.
Conclusion: Our processing pipeline offers a reliable method for assessing spinal cord compression in DCM patients. Normalized spinal cord morphometrics, particularly the CSA could have potential for monitoring DCM disease severity and progression, guiding treatment decisions. Furthermore, to our knowledge our study is the first to apply the generic spinal cord acquisition protocol, ensuring consistent imaging across different MRI scanners and settings. Coupled with our semiautomated analysis pipeline, this protocol is key for the detailed morphometric characterization of compressed spinal cords in patients with DCM, a disease that is both complex and heterogenous. This study was funded by the National Institute of Neurological Disorders and Stroke (NINDS) (K23:NS091430) and (R01: NS129852-01A1).
Keywords: Atrophy; Degenerative cervical myelopathy; Generic spine protocol; MRI; Spinal Cord Toolbox; Spinal cord.
Copyright © 2024 Elsevier Inc. All rights reserved.
Conflict of interest statement
Declaration of competing interest One or more of the authors declare financial or professional relationships on ICMJE-TSJ disclosure forms.
Figures


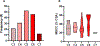

References
-
- Cloney MB, Smith ZA, Weber KA 2nd, Parrish TB. Quantitative Magnetization Transfer MRI Measurements of the Anterior Spinal Cord Region are Associated With Clinical Outcomes in Cervical Spondylotic Myelopathy. Spine (Phila Pa 1976). May 15 2018;43(10):675–680. doi: 10.1097/brs.0000000000002470 - DOI - PMC - PubMed
MeSH terms
Grants and funding
LinkOut - more resources
Full Text Sources
Medical
Miscellaneous

