Assessment of urinary 6-oxo-pipecolic acid as a biomarker for ALDH7A1 deficiency
- PMID: 39038845
- PMCID: PMC11670438
- DOI: 10.1002/jimd.12783
Assessment of urinary 6-oxo-pipecolic acid as a biomarker for ALDH7A1 deficiency
Abstract
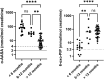
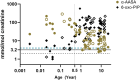
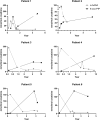
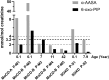
ALDH7A1 deficiency is an epileptic encephalopathy whose seizures respond to treatment with supraphysiological doses of pyridoxine. It arises as a result of damaging variants in ALDH7A1, a gene in the lysine catabolism pathway. α-Aminoadipic semialdehyde (α-AASA) and Δ1-piperideine-6-carboxylate (P6C), which accumulate because of the block in the lysine pathway, are diagnostic biomarkers for this disorder. Recently, it has been reported that 6-oxo-pipecolic acid (6-oxo-PIP) also accumulates in the urine, CSF and plasma of ALDH7A1-deficient individuals and that, given its improved stability, it may be a more suitable biomarker for this disorder. This study measured 6-oxo-PIP in urine from a cohort of 30 patients where α-AASA was elevated and showed that it was above the normal range in all those above 6 months of age. However, 6-oxo-PIP levels were within the normal range in 33% of the patients below 6 months of age. Levels increased with age and correlated with a decrease in α-AASA levels. Longitudinal analysis of urine samples from ALDH7A1-deficient patients who were on a lysine restricted diet whilst receiving supraphysiological doses of pyridoxine showed that levels of 6-oxo-PIP remained elevated whilst α-AASA decreased. Similar to α-AASA, we found that elevated urinary excretion of 6-oxo-PIP can also occur in individuals with molybdenum cofactor deficiency. This study demonstrates that urinary 6-oxo-PIP may not be a suitable biomarker for ALDH7A1 deficiency in neonates. However, further studies are needed to understand the biochemistry leading to its accumulation and its potential long-term side effects.
Keywords: 6‐oxo‐pipecolic acid; ALDH7A1 deficiency; aminoadipic semialdehyde; piperideine‐6‐carboxylate; pyridoxine‐dependent epilepsy.
© 2024 The Author(s). Journal of Inherited Metabolic Disease published by John Wiley & Sons Ltd on behalf of SSIEM.
Conflict of interest statement
Youssef Khalil, Emma Footitt, Reddy Vootukuri, Spyros Batzios, Matthew P. Wilson, Viktor Kožich, Peter T. Clayton and Philippa B. Mills declare no conflicts of interest. Michael F. Wempe and Curtis R. Coughlin II are named as inventors for a patent describing 6‐oxo‐pipecolic acid quantitation by mass spectrometry (US‐20220057371‐A1).
Figures


References
-
- Mills PB, Struys E, Jakobs C, et al. Mutations in antiquitin in individuals with pyridoxine‐dependent seizures. Nat Med. 2006;12(3):307‐309. - PubMed
-
- Bok LA, Halbertsma FJ, Houterman S, et al. Long‐term outcome in pyridoxine‐dependent epilepsy. Dev Med Child Neurol. 2012;54(9):849‐854. - PubMed
-
- Coughlin CR II, Tseng LA, Abdenur JE, et al. Consensus guidelines for the diagnosis and management of pyridoxine‐dependent epilepsy due to alpha‐aminoadipic semialdehyde dehydrogenase deficiency. J Inherit Metab Dis. 2021;44(1):178‐192. - PubMed
-
- Mills PB, Footitt EJ, Ceyhan S, et al. Urinary AASA excretion is elevated in patients with molybdenum cofactor deficiency and isolated sulphite oxidase deficiency. J Inherit Metab Dis. 2012;35(6):1031‐1036. - PubMed
-
- Struys EA, Nota B, Bakkali A, Al Shahwan S, Salomons GS, Tabarki B. Pyridoxine‐dependent epilepsy with elevated urinary alpha‐amino adipic semialdehyde in molybdenum cofactor deficiency. Pediatrics. 2012;130(6):e1716‐e1719. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Miscellaneous

