Establishment of a multisite umbrella cohort study protocol to describe the epidemiology and aetiologies of acute undifferentiated febrile illness in Latin America
- PMID: 39038857
- PMCID: PMC11404142
- DOI: 10.1136/bmjopen-2023-083560
Establishment of a multisite umbrella cohort study protocol to describe the epidemiology and aetiologies of acute undifferentiated febrile illness in Latin America
Abstract
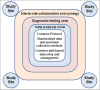
Introduction: Acute undifferentiated febrile illnesses (AUFIs) impose a large burden in the tropics. Understanding of AUFI's epidemiology is limited. Insufficient diagnostic capacity hinders the detection of outbreaks. The lack of interconnection in healthcare systems hinders timely response. We describe a protocol to study the epidemiology and aetiologies of AUFI and pathogen discovery in strategic areas of Latin America (LA).
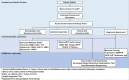
Methods and analysis: Global Infectious Diseases Network investigators comprising institutions in Colombia, Dominican Republic, México, Perú and the USA, developed a common cohort study protocol. The primary objective is to determine the aetiologies of AUFI at healthcare facilities in high-risk areas. Data collection and laboratory testing for viral, bacterial and parasitic agents are performed in rural and urban healthcare facilities and partner laboratories. Centralised laboratory and data management cores deploy diagnostic tests and data management tools. Subjects >6 years with fever for <8 days without localised infection are included in the cohort. They are evaluated during the acute and convalescent phases of illness. Study personnel collect clinical and epidemiological information. Blood, urine, nasal or pharyngeal swabs and saliva are collected in the acute phase and blood in convalescent phase. Specimens are banked at -80°C. Malaria, dengue and COVID-19 are tested onsite in the acute phase. The acute-phase serum is PCR tested for dengue, chikungunya, Venezuelan equine encephalitis, Mayaro, Oropouche, Zika, and yellow fever viruses. Paired convalescent and acute serum antibody titters are tested for arbovirus, Leptospira spp, and Rickettsia spp. Serum is used for viral cultures and next-generation sequencing for pathogen discovery. Analysis includes variable distributions, risk factors and regression models. Laboratory results are shared with health authorities and network members.
Ethics and dissemination: The protocol was approved by local ethics committees and health authorities. The results will be published in peer-reviewed journals. All study results are shared with local and regional health authorities.
Keywords: epidemiology; neglected diseases; public health; tropical medicine; virology.
© Author(s) (or their employer(s)) 2024. Re-use permitted under CC BY-NC. No commercial re-use. See rights and permissions. Published by BMJ.
Conflict of interest statement
Competing interests: None declared.
Figures
References
Publication types
MeSH terms
Grants and funding
LinkOut - more resources
Full Text Sources
Medical
Miscellaneous
