Senescent cancer-associated fibroblasts in pancreatic adenocarcinoma restrict CD8+ T cell activation and limit responsiveness to immunotherapy in mice
- PMID: 39039076
- PMCID: PMC11263607
- DOI: 10.1038/s41467-024-50441-7
Senescent cancer-associated fibroblasts in pancreatic adenocarcinoma restrict CD8+ T cell activation and limit responsiveness to immunotherapy in mice
Abstract
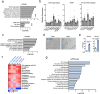
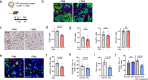
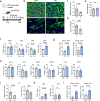
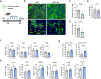
Senescent cells within tumors and their stroma exert complex pro- and anti-tumorigenic functions. However, the identities and traits of these cells, and the potential for improving cancer therapy through their targeting, remain poorly characterized. Here, we identify a senescent subset within previously-defined cancer-associated fibroblasts (CAFs) in pancreatic ductal adenocarcinomas (PDAC) and in premalignant lesions in mice and humans. Senescent CAFs isolated from mouse and humans expressed elevated levels of immune-regulatory genes. Depletion of senescent CAFs, either genetically or using the Bcl-2 inhibitor ABT-199 (venetoclax), increased the proportion of activated CD8+ T cells in mouse pancreatic carcinomas, whereas induction of CAF senescence had the opposite effect. Combining ABT-199 with an immune checkpoint therapy regimen significantly reduced mouse tumor burden. These results indicate that senescent CAFs in PDAC stroma limit the numbers of activated cytotoxic CD8+ T cells, and suggest that their targeted elimination through senolytic treatment may enhance immunotherapy.
© 2024. The Author(s).
Conflict of interest statement
B.Z.S. receives sponsored research support from Boehringer-Ingelheim and Revolution Medicines and holds equity in iTeos Therapeutics. T.G. Receives research support from Astra Zeneca, Abbvie honoraria, consultation fees from Abbvie and MSD Merck, royalties and consultant fees from Curesponse, and speaker fees from Abbvie and ClearNote Health. The remaining authors declare no competing interests.
Figures



References
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical
Molecular Biology Databases
Research Materials

