Cell autonomous functions of CD47 in regulating cellular plasticity and metabolic plasticity
- PMID: 39039207
- PMCID: PMC11445524
- DOI: 10.1038/s41418-024-01347-w
Cell autonomous functions of CD47 in regulating cellular plasticity and metabolic plasticity
Abstract
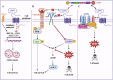
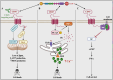
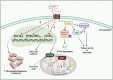
CD47 is a ubiquitously expressed cell surface receptor, which is widely known for preventing macrophage-mediated phagocytosis by interacting with signal regulatory protein α (SIRPα) on the surface of macrophages. In addition to its role in phagocytosis, emerging studies have reported numerous noncanonical functions of CD47 that include regulation of various cellular processes such as proliferation, migration, apoptosis, differentiation, stress responses, and metabolism. Despite lacking an extensive cytoplasmic signaling domain, CD47 binds to several cytoplasmic proteins, particularly upon engaging with its secreted matricellular ligand, thrombospondin 1. Indeed, the regulatory functions of CD47 are greatly influenced by its interacting partners. These interactions are often cell- and context-specific, adding a further level of complexity. This review addresses the downstream cell-intrinsic signaling pathways regulated by CD47 in various cell types and environments. Some of the key pathways modulated by this receptor include the PI3K/AKT, MAPK/ERK, and nitric oxide signaling pathways, as well as those implicated in glucose, lipid, and mitochondrial metabolism. These pathways play vital roles in maintaining tissue homeostasis, highlighting the importance of understanding the phagocytosis-independent functions of CD47. Given that CD47 expression is dysregulated in a variety of cancers, improving our understanding of the cell-intrinsic signals regulated by this molecule will help advance the development of CD47-targeted therapies.
© 2024. The Author(s).
Conflict of interest statement
The authors declare no competing interests.
Figures


References
-
- Rebres RA, Vaz LE, Green JM, Brown EJ. Normal ligand binding and signaling by CD47 (integrin-associated protein) requires a long range disulfide bond between the extracellular and membrane-spanning domains. J Biol Chem. 2001;276:34607–16. - PubMed
-
- Mushegian A. Refining structural and functional predictions for secretasome components by comparative sequence analysis. Proteins. 2002;47:69–74. - PubMed
-
- Reinhold MI, Lindberg FP, Plas D, Reynolds S, Peters MG, Brown EJ. In vivo expression of alternatively spliced forms of integrin-associated protein (CD47). J Cell Sci. 1995;108:3419–25. - PubMed
-
- Lee EH, Hsieh YP, Yang CL, Tsai KJ, Liu CH. Induction of integrin-associated protein (IAP) mRNA expression during memory consolidation in rat hippocampus. Eur J Neurosci. 2000;12:1105–12. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Research Materials
Miscellaneous

