Targeting monocytic Occludin impairs transendothelial migration and HIV neuroinvasion
- PMID: 39039298
- PMCID: PMC11315906
- DOI: 10.1038/s44319-024-00190-x
Targeting monocytic Occludin impairs transendothelial migration and HIV neuroinvasion
Abstract
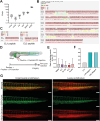
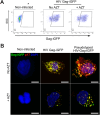
Transmigration of circulating monocytes from the bloodstream to tissues represents an early hallmark of inflammation. This process plays a pivotal role during viral neuroinvasion, encephalitis, and HIV-associated neurocognitive disorders. How monocytes locally unzip endothelial tight junction-associated proteins (TJAPs), without perturbing impermeability, to reach the central nervous system remains poorly understood. Here, we show that human circulating monocytes express the TJAP Occludin (OCLN) to promote transmigration through endothelial cells. We found that human monocytic OCLN (hmOCLN) clusters at monocyte-endothelium interface, while modulation of hmOCLN expression significantly impacts monocyte transmigration. Furthermore, we designed OCLN-derived peptides targeting its extracellular loops (EL) and show that transmigration of treated monocytes is inhibited in vitro and in zebrafish embryos, while preserving vascular integrity. Monocyte transmigration toward the brain is an important process for HIV neuroinvasion and we found that the OCLN-derived peptides significantly inhibit HIV dissemination to cerebral organoids. In conclusion, our study identifies an important role for monocytic OCLN during transmigration and provides a proof-of-concept for the development of mitigation strategies to prevent monocyte infiltration and viral neuroinvasion.
Keywords: AIDS; Central Nervous System; Endothelial Cells; Tight Junction; Zebrafish.
© 2024. The Author(s).
Conflict of interest statement
The authors declare no competing interests.
Figures







References
-
- Berre S, Gaudin R, Cunha de Alencar B, Desdouits M, Chabaud M, Naffakh N, Rabaza-Gairi M, Gobert FX, Jouve M, Benaroch P (2013) CD36-specific antibodies block release of HIV-1 from infected primary macrophages and its transmission to T cells. J Exp Med 210:2523–2538 10.1084/jem.20130566 - DOI - PMC - PubMed
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical
Molecular Biology Databases
Miscellaneous

