ENDOLUNG trial. A phase 1/2 study of the Akt/mTOR inhibitor and autophagy inducer Ibrilatazar (ABTL0812) in combination with paclitaxel/carboplatin in patients with advanced/recurrent endometrial cancer
- PMID: 39039449
- PMCID: PMC11265130
- DOI: 10.1186/s12885-024-12501-5
ENDOLUNG trial. A phase 1/2 study of the Akt/mTOR inhibitor and autophagy inducer Ibrilatazar (ABTL0812) in combination with paclitaxel/carboplatin in patients with advanced/recurrent endometrial cancer
Erratum in
-
Correction: ENDOLUNG trial. A phase 1/2 study of the Akt/mTOR inhibitor and autophagy inducer Ibrilatazar (ABTL0812) in combination with paclitaxel/carboplatin in patients with advanced/recurrent endometrial cancer : Alexandra Leary.BMC Cancer. 2024 Jul 29;24(1):911. doi: 10.1186/s12885-024-12700-0. BMC Cancer. 2024. PMID: 39075404 Free PMC article. No abstract available.
Abstract
Background: Carboplatin and paclitaxel (CP) have been the standard of care for advanced/recurrent endometrial cancer (EC) for many years. However, this chemotherapy combination shows limited efficacy and recurrences often occur in less than 12 months. ABTL0812 is a novel drug that selectively kill cancer cells by cytotoxic autophagy and has shown anticancer efficacy in preclinical models of EC in combination with CP.
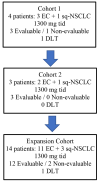
Methods: ENDOLUNG was an open-label, phase 1/2 clinical trial designed to determine the safety and efficacy of Ibrilatazar (ABTL0812) with CP in patients with advanced/recurrent EC and non-irradiable stage III and IV squamous non-small cell lung cancer (sq-NSCLC). The phase 1 part consisted of a 3 + 3 de-escalation design followed by an expansion cohort with 12 patients. The primary endpoint was safety. ABTL0812 starting dose was 1300 mg tid combined with carboplatin at area under the curve (AUC) 5 and paclitaxel at 175 mg/m2 both administered every 21 days for up to 8 cycles. The phase 2 part included a total of 51 patients. The primary endpoint was overall response rate (ORR) and the secondary endpoints included duration of response (DOR), progression-free survival (PFS) and overall survival (OS).
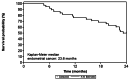
Results: During the phase 1 only one dose limiting toxicity (DLT), a grade 4 neutropenia, was observed in 1 out of 6 patients, thus no de-escalation was applied. One additional DLT, a grade 3 febrile neutropenia, was observed in the expansion cohort, thus the recommended phase 2 dose (RP2D) for ABTL0812 was established at 1300 mg tid. Most frequent hematological adverse events (AE) of the combination were neutropenia (52.9%), anemia (37.3%) and thrombocytopenia (19.6%). Nausea (66.7%), asthenia (66.7%), diarrhea (54.9%) and vomiting (54.9%) were the most frequent non-hematological adverse events (AEs). The combination of ABTL0812 plus CP showed an ORR of 65.8% (13.2% complete response and 52.6% partial response) with a median DOR of 7.4 months (95% CI: 6.3-10.8 months). Median PFS was 9.8 months (95% CI: 6.6-10.6) and median OS 23.6 months (95% CI 6.4-ND). Pharmacokinetic parameters were compatible with target engagement observed in preclinical studies, and blood pharmacodynamic biomarkers indicated sustained target regulation during, at least, 28 days after starting the treatment.
Conclusions: This study suggests that the combination of ABTL0812 with CP is safe and feasible with an encouraging activity in patients with advanced/recurrent EC. Our data warrant further confirmation in prospective randomized trials.
Trial registration: EU Clinical Trial Register, EudraCT number 2016-001352-21 and National Clinical Trials Number, NCT03366480. Registration on 19 September 2016.
Keywords: Autophagy; Chemotherapy; Endometrial cancer; Phase 1/2; Safety profile.
© 2024. The Author(s).
Conflict of interest statement
The study was initially registered on 19/09/2016 and approved in Spain by the National Competent Authority (AEMPS: Agencia Española de Medicamentos y Productos Sanitarios) and by the Ethics Committee “Comité de Ética de la Investigación con medicamentos (CEIm)” from Hospital Universitari Vall d’Hebrón (Barcelona, Spain), and in France by the National Competent Authority (ANSM: Agence nationale de sécurité du médicament et des produits de santé) and by the Ethics Committee “Comité de Protection des Personnes SUD-EST II” from Groupemet Hospitalier Est (Bron, France). The study was performed in accordance with the Declaration of Helsinki, the International Conference on Harmonisation of Technical Requirements for Pharmaceuticals for Human Use, Good Clinical Practice, and local laws. All patients provided written informed consent. The trial is registered with the USA Registry of Clinical Trials (ClinicalTrials.gov) with the trial ID number NCT03366480. All other authors declare no competing interests.
A.L. declares advisory board fees and travel support from AstraZeneca, advisory board fees from Clovis, Gridstone, AbilityPharma and Pfizer, grant support and advisory board fees from GamaMabs, and grant support from Merus and Sanofi. R.S. declares research grants from Astrazeneca, consulting fees from GSK, Seagen, Eisai, Novartis, and Clovis Oncology, and non-financial support from MSD, Novartis, and GSK. I.R.C. declares self-honoraria from Agenus, Blueprint, BMS, PharmaMar, Genmab, Pfizer, AstraZeneca, Roche, GSK, MSD, Deciphera, Mersena, Merck Sereno, Novartis, Amgen, Macrogenics, Tesaro and Clovis and consulting fees from Abbvie, Agenus, Advaxis, BMS, ESAÏ, Daichi, PharmaMar, Genmab, Pfizer, AstraZeneca, Roche/Genentech, GSK, MSD, Deciphera, Mersana, Merck Sereno, Novartis, Amgen, Tesaro and Clovis; research grant/funding (self) from MSD, Roche and BMS and other institution fees from GSK, MSD, Roche, BMS and Astrazeneca. M.R. declares advisory board GSK, speaker bureau AZ, travel grants AZ, MSD, dedication activities AZP.B.G. declares advisory Board and travel expenses from: Astra Zeneca, Clovis, GSK, MSD, Eisai and Pharmamar M.G.M. has nothing to disclose related to this job. Speaking: MSD, Astra Zeneca; Registration and attending scientific meetings: MSD, Clovis, GSKE.G. declares consulting fees from Roche, Ellipses Pharma, Boehringer Ingelheim, Janssen Global Services, Seattle Genetics, Thermo Fisher, MabDiscovery, Anaveon, F-Star Therapeutics, Hengrui, Sanofi, Incyte, Medscape. Grants or contracts from Novartis, Roche, Thermo Fisher, AstraZeneca, Taiho, BeiGene and Janssen. Payment or honoraria for lectures, presentations, speakers bureaus, manuscript writing or educational events from Merck Sharp & Dohme, Roche, Thermo Fisher, Novartis and SeaGen. Holds stocks from 1TRIALSP. Is employed by NEXT Oncology and PI or Co-PI institutional from Adaptimmune LLC, Affimed Gmbh, Amgen SA, Anaveon AG, AstraZeneca AB, Bicycletx Ltd, BioInvent International AB, Biontech SE, Biontech Small Molecules Gmbh, Boehringer Ingelhem International Gmbh, Catalym Gmbh, Cyclacel Biopharmaceuticals, Cytovation AS, Cytomx, F.Hoffmann La Roche Ltd, F-Star Beta Limited, Genentech Inc, Genmab B.V., Hifibio Therapeutics, Hutchison Medipharma Limited, Icon, Imcheck Therapeutics, Immunocore Ltd, Incyte Corporation, Incyte Europe Sàrl, Janssen-Cilag International NV, Janssen-Cilag SA, Laboratorios Servier SL, Medimmune Llc, Merck & Co, Inc, Merck Kgga, Novartis Farmacéutica, S.A, Peptomyc, Pfizer Slu, Relay Therapeutics, Replimmune, Ribon Therapeutics, Ryvu Therapeutics SA, Seattle Genetics Inc, Sotio AS, Sqz Biotechnologies, Symphogen A/S, Taiho Pharma Usa Inc and T-Knife GmbhJ.B.B. has received consulting fees and honoraria from Roche, Pfizer, AstraZeneca, Vifor, Sanofi, MSD and BMS. T.M. declares consulting/advisory role fees by Roche, Bristol Myers, Boeringher, Astra Zeneca. Research Funding Grant by Kyowa Kirin and Janssen, all of them unrelated with the current workE.N. declares the following financial Interests: Invited Speaker, Personal from Amgen, AstraZeneca, Bristol Myers Squibb, Illumina, Daiichi, Janssen, Lilly, Merck Sharp Dohme, Pfizer, Qiagen, Roche, Sanofi, Takeda; Advisory Board, Personal from Amgen, AstraZeneca, Boehringer, Ingelheim, Bristol Myers Squibb, Daiichi SankyoJanssen, Lilly, Merck Serono, Merck Sharp Dohme, Pfizer, Pierre Fabre, Regeneron, Roche, Sanofi, Takeda and Qiagen. Funding, Personal and Institutional, No financial interest from Bristol Myers Squibb (funded a clinical trial), Merck Serono (funded a clinical trial) and Roche (funded a clinical trial). Non-Financial Interests, Advisory Role from Apollomics, Pfizer and Roche. Member of the Steering Committee of the Spanish Lung Cancer Group (GECP). J.R. reports non-financial support and reasonable reimbursement for travel from European Society for Medical Oncology and Loxo Oncology; receiving consulting and travel fees from Ellipses Pharma, Molecular Partners, IONCTURA, Sardona, Mekanistic, Amgen, Merus, MonteRosa, Aadi and Bridgebio (including serving on the scientific advisory board); Consulting fees from Vall d’Hebron Institute of Oncology/Ministero De Empleo Y Seguridad Social, Chinese University of Hong Kong, Boxer Capital, LLC, Tang Advisors, LLC and Guidepoint, receiving research funding from Blueprint Medicines, Merck Sharp & Dohme, Hummingbird, AstraZenneca, Yingli, Vall d’Hebron Institute of Oncology/Cancer Core Europe; and serving as investigator in clinical trials with Cancer Core Europe, Symphogen, BioAlta, Pfizer, Kelun-Biotech, GlaxoSmithKline, Taiho, Roche Pharmaceuticals, Hummingbird, Yingli, Bicycle Therapeutics, Merus, AadiBioscience, ForeBio, Loxo Oncology, Hutchinson MediPharma, Ideaya, Amgen, Tango Therapeutics, Mirati, Linnaeus Therapeutics, MonteRosa, Kinnate, Yingli, Debio, BioTheryX, Storm Therapeutics, Beigene, MapKure, Relay, Novartis, FusionPharma, C4 Therapeutics, Scorpion Therapeutics, Incyte, Fog Pharmaceuticals, Tyra, Nuvectis Pharma. J.M.L. declares to be the chairman of the Scientific Advisory Board from AbilityPharma, and that holds stocks from Ability Pharmaceuticals P.M.G. declares that was an employee of Ability Pharmaceuticals and holds stocks from the company G.F.D. declares that is an employee of Ability Pharmaceuticals and holds stocks from the company O.P.G. declares that is an employee of Ability PharmaceuticalsH.P.M. declares that is an employee of Ability Pharmaceuticals and holds stocks from the company M.Y.V. declares that was an employee of Ability Pharmaceuticals and holds stocks from the company M.C. declares that is an employee of Ability Pharmaceuticals and holds stocks from the company A.P.C declares that is the chairman of the Clinical Advisory Board of Ability Pharmaceuticals and that holds stocks from the companyJ.A. declares that was an employee of Ability Pharmaceuticals and holds stocks from the company * C.D. declares that is the CEO, CSO and co-founder of Ability Pharmaceuticals and that holds stocks from the companyA.P.F. declares speaker bureau from Astrazeneca, Clovis, GSK, Pharmamar, MSD, Pharma&. Advisory from Astrazeneca, Eisai, MSD, AbilityPharma, Pharmamar, Clovis. Travel expenses from Astrazeneca, Pharmamar, GSK. Grants to my institution from Astrazeneca, GSK and Pharmamar. A.O. reports personal fees for advisory board membership from Agenus, AstraZeneca, Clovis Oncology, Corcept Therapeutics, Deciphera Pharmaceuticals, Eisai, Exelisis, EMD Serono, F. Hoffmann-La Roche, Genmab, GSK, ImmunoGen, Itheos, Merck Sharps & Dohme de España, SA, Mersana Therapeutics, Novocure, OneXerna Therapeutics, Inc., PharmaMar, Regeneron, Sattucklabs, Seagen and Sutro Biopharma; personal fees for travel/accommodation from AstraZeneca, PharmaMar and Roche; institutional funding from Abbvie Deutschland, Advaxis Inc., Aeterna Zentaris, Amgen, Aprea Therapeutics AB, Bristol Myers Squibb, Clovis Oncology Inc, EISAI limited LTD, F. Hoffmann –La Roche LTD, Immunogen Inc, Merck, Sharp & Dohme de España SA, Millennium Pharmaceuticals Inc, PharmaMar SA, Regeneron Pharmaceuticals and Tesaro Inc.; non-remunerated roles at ESMO (member, Officer, Co-Chair of the ESMO Gynaecological Cancers Congress 2023-2025, Chair of the Gynaecological Track ESMO 2019, Scientific Track Member Gynaecological Cancers ESMO 2018, ESMO 2020, ESMO 2022, member of the Gynaecological Cancers Faculty and Subject Editor for the Gynaecological Clinical Practice Guidelines); a non-remunerated role at GCIG (member and Cervix Cancer Chair on behalf of GEICO); and membership of ASCO, GOG and SEOM.All other authors do not have any kind of competing interest.
Figures




References
-
- Oaknin A, Bosse TJ, Creutzberg CL, Giornelli G, Harter P, Joly F et al. Endometrial cancer: ESMO clinical practice guideline for diagnosis, treatment and follow-up. Ann Oncol. 2022;S0923–7534(22)01207–8. - PubMed
Publication types
MeSH terms
Substances
Associated data
Grants and funding
LinkOut - more resources
Full Text Sources
Medical
Miscellaneous

