Initial AXL and MCL-1 inhibition contributes to abolishing lazertinib tolerance in EGFR-mutant lung cancer cells
- PMID: 39039802
- PMCID: PMC11447890
- DOI: 10.1111/cas.16292
Initial AXL and MCL-1 inhibition contributes to abolishing lazertinib tolerance in EGFR-mutant lung cancer cells
Abstract
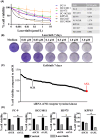
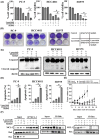
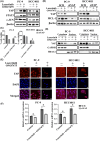
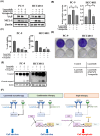
Lazertinib, a novel third-generation epidermal growth factor receptor tyrosine kinase inhibitor (EGFR-TKI), demonstrates marked efficacy in EGFR-mutant lung cancer. However, resistance commonly develops, prompting consideration of therapeutic strategies to overcome initial drug resistance mechanisms. This study aimed to elucidate the adaptive resistance to lazertinib and advocate novel combination treatments that demonstrate efficacy in preventing resistance as a first-line treatment for EGFR mutation-positive NSCLC. We found that AXL knockdown significantly inhibited lung cancer cell viability in the presence of lazertinib, indicating that AXL activation contributes to lazertinib resistance. However, long-term culture with a combination of lazertinib and AXL inhibitors led to residual cell proliferation and increased the MCL-1 expression level, which was mediated by the nuclear translocation of the transcription factor YAP. Triple therapy with an MCL-1 or YAP inhibitor in combination with lazertinib and an AXL inhibitor significantly reduced cell viability and increased the apoptosis rate. These results demonstrate that AXL and YAP/MCL-1 signals contribute to adaptive lazertinib resistance in EGFR-mutant lung cancer cells, suggesting that the initial dual inhibition of AXL and YAP/MCL-1 might be a highly effective strategy in eliminating lazertinib-resistant cells.
Keywords: AXL; EGFR; EGFR‐TKI; MCL‐1; YAP.
© 2024 The Author(s). Cancer Science published by John Wiley & Sons Australia, Ltd on behalf of Japanese Cancer Association.
Conflict of interest statement
T. Yamada received speaking honoraria from Eli Lilly. T. Yamada received commercial research grants from Pfizer, Ono Pharmaceutical, Janssen Pharmaceutical K.K., AstraZeneca, and Takeda Pharmaceutical Company Limited. K. Takayama received research grants from Chugai‐Roche Co. and Ono Pharmaceutical Co. and personal fees from AstraZeneca Co., Chugai‐Roche Co., MSD‐Merck Co., Eli Lilly Co., Boehringer‐Ingelheim Co., and Daiichi‐Sankyo Co. The other authors have no conflict of interest.
Figures


Similar articles
-
ONO-7475, a Novel AXL Inhibitor, Suppresses the Adaptive Resistance to Initial EGFR-TKI Treatment in EGFR-Mutated Non-Small Cell Lung Cancer.Clin Cancer Res. 2020 May 1;26(9):2244-2256. doi: 10.1158/1078-0432.CCR-19-2321. Epub 2020 Jan 17. Clin Cancer Res. 2020. PMID: 31953310
-
AXL degradation in combination with EGFR-TKI can delay and overcome acquired resistance in human non-small cell lung cancer cells.Cell Death Dis. 2019 May 1;10(5):361. doi: 10.1038/s41419-019-1601-6. Cell Death Dis. 2019. PMID: 31043587 Free PMC article.
-
MET and AXL inhibitor NPS-1034 exerts efficacy against lung cancer cells resistant to EGFR kinase inhibitors because of MET or AXL activation.Cancer Res. 2014 Jan 1;74(1):253-62. doi: 10.1158/0008-5472.CAN-13-1103. Epub 2013 Oct 28. Cancer Res. 2014. PMID: 24165158
-
Strategies to overcome acquired resistance to EGFR TKI in the treatment of non-small cell lung cancer.Clin Transl Oncol. 2019 Oct;21(10):1287-1301. doi: 10.1007/s12094-019-02075-1. Epub 2019 Mar 12. Clin Transl Oncol. 2019. PMID: 30864018 Review.
-
Epidermal Growth Factor Receptor (EGFR) Pathway, Yes-Associated Protein (YAP) and the Regulation of Programmed Death-Ligand 1 (PD-L1) in Non-Small Cell Lung Cancer (NSCLC).Int J Mol Sci. 2019 Aug 5;20(15):3821. doi: 10.3390/ijms20153821. Int J Mol Sci. 2019. PMID: 31387256 Free PMC article. Review.
Cited by
-
Identification and verification of immune and oxidative stress-related diagnostic indicators for malignant lung nodules through WGCNA and machine learning.Sci Rep. 2025 Jul 1;15(1):22449. doi: 10.1038/s41598-025-04639-4. Sci Rep. 2025. PMID: 40594252 Free PMC article.
-
Recent advances in TAM mechanisms in lung diseases.J Transl Med. 2025 Apr 26;23(1):479. doi: 10.1186/s12967-025-06398-2. J Transl Med. 2025. PMID: 40287707 Free PMC article. Review.
References
-
- Siegel RL, Miller KD, Wagle NS, Jemal A. Cancer statistics, 2023. CA Cancer J Clin. 2023;73:17‐48. - PubMed
-
- Govindan R, Page N, Morgensztern D, et al. Changing epidemiology of small‐cell lung cancer in the United States over the last 30 years: analysis of the surveillance, epidemiologic, and end results database. J Clin Oncol. 2006;24:4539‐4544. - PubMed
-
- Recondo G, Facchinetti F, Olaussen KA, Besse B, Friboulet L. Making the first move in EGFR‐driven or ALK‐driven NSCLC: first‐generation or nextgeneration TKI? Nat Rev Clin Oncol. 2018;15:694‐708. - PubMed
-
- Papadimitrakopoulou VA, Mok TS, Han JY, et al. Osimertinib versus platinum‐pemetrexed for patients with EGFR T790M advanced NSCLC and progression on a prior EGFR‐tyrosine kinase inhibitor: AURA3 overall survival analysis. Ann Oncol. 2020;31:1536‐1544. - PubMed
-
- Ramalingam SS, Vansteenkiste J, Planchard D, et al. Overall survival with osimertinib in untreated, EGFR‐mutated advanced NSCLC. N Engl J Med. 2020;382:41‐50. - PubMed
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Medical
Research Materials
Miscellaneous

