Effect of 2 Forms of Tenofovir on Duodenal Enterocytes-A Hypothesis for Different Effect of Tenofovir Disoproxil Fumarate and Tenofovir Alafenamide on Body Weight and Plasma Lipids
- PMID: 39039812
- PMCID: PMC11848257
- DOI: 10.1093/cid/ciae374
Effect of 2 Forms of Tenofovir on Duodenal Enterocytes-A Hypothesis for Different Effect of Tenofovir Disoproxil Fumarate and Tenofovir Alafenamide on Body Weight and Plasma Lipids
Abstract
Background: Tenofovir disoproxil fumarate (TDF), compared to tenofovir alafenamide (TAF), leads to lower body weight and plasma lipids by an unknown mechanism. We hypothesize that TDF, when absorbed, may damage enterocytes of the proximal duodenum, leading to reduced absorption of nutrients.
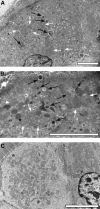
Methods: People with human immunodeficiency virus, without significant gastrointestinal symptoms, receiving a regimen containing TDF (n = 12) or TAF (n = 12), underwent esophagogastroduodenoscopies. Plasma/serum concentrations of nutrients absorbed from proximal duodenum and serum intestinal fatty acid-binding protein (I-FABP), a marker of enterocyte damage, were measured. Cytochrome c oxidase/succinate dehydrogenase (COX/SDH) staining and electron microscopy (EM) were conducted to evaluate mitochondria.
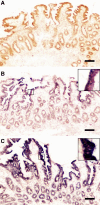
Results: Five patients in the TDF group (1 celiac disease [excluded from further analyses], 1 Helicobacter gastritis, and 3 esophagitis) and 2 in the TAF group (2 esophagitis) had a pathological finding in esophagogastroduodenoscopy. Villi were flatter (337 [59] vs 397 [42] μm; P = .016), crypts nonsignificantly deeper (200 [46] vs 176 [27] μm; P = .2), and villus-to-crypt ratio lower (1.5 [0.42] vs 2.5 [0.51]; P = .009) in the TDF versus TAF group (mean [standard deviation]). I-FABP concentration was higher in the TDF versus TAF group (3.0 [1.07] vs 1.8 [0.53] ng/mL; P = .003). The TDF group had numerically but not statistically significantly lower concentrations of folate and vitamins A, B1, D, and E. COX/SDH staining and EM showed similar mitochondrial damage in both groups.
Conclusions: Duodenal villous alterations may explain TDF-associated decrease in body weight and plasma lipids. Larger studies are needed to evaluate concentrations of nutrients absorbed from duodenum among TDF users..
Clinical trials registration: NCT05326971; EudraCT 2022-000849.
Keywords: duodenal villus; enterocyte; intestinal fatty acid–binding protein; tenofovir alafenamide; tenofovir disoproxil fumarate.
© The Author(s) 2024. Published by Oxford University Press on behalf of Infectious Diseases Society of America.
Conflict of interest statement
Potential conflicts of interest. I. A. has received honoraria, lecture fees, and conference support from Gilead, Merck, and GSK/ViiV, and a research grant from Gilead. N. S. has received lecture fees from Bristol Myers Squibb and consulting fees from Aiforia Technologies Plc. J. S. has received honoraria, lecture fees, and conference support from Gilead, Merck, and GSK/ViiV, and research grants from Gilead and Merck. All other authors report no potential conflicts. All authors have submitted the ICMJE Form for Disclosure of Potential Conflicts of Interest. Conflicts that the editors consider relevant to the content of the manuscript have been disclosed.
Figures
Similar articles
-
Plasma concentration of neurofilament light chain protein decreases after switching from tenofovir disoproxil fumarate to tenofovir alafenamide fumarate.PLoS One. 2019 Dec 11;14(12):e0226276. doi: 10.1371/journal.pone.0226276. eCollection 2019. PLoS One. 2019. PMID: 31826005 Free PMC article. Clinical Trial.
-
A retrospective analysis of weight changes in HIV-positive patients switching from a tenofovir disoproxil fumarate (TDF)- to a tenofovir alafenamide fumarate (TAF)-containing treatment regimen in one German university hospital in 2015-2017.Infection. 2019 Feb;47(1):95-102. doi: 10.1007/s15010-018-1227-0. Epub 2018 Sep 29. Infection. 2019. PMID: 30269210 Free PMC article.
-
Efficacy and safety of the regimens containing tenofovir alafenamide versus tenofovir disoproxil fumarate in fixed-dose single-tablet regimens for initial treatment of HIV-1 infection: A meta-analysis of randomized controlled trials.Int J Infect Dis. 2020 Apr;93:108-117. doi: 10.1016/j.ijid.2020.01.035. Epub 2020 Jan 25. Int J Infect Dis. 2020. PMID: 31988012 Review.
-
Weight and Metabolic Changes After Switching From Tenofovir Disoproxil Fumarate to Tenofovir Alafenamide in People Living With HIV : A Cohort Study.Ann Intern Med. 2021 Jun;174(6):758-767. doi: 10.7326/M20-4853. Epub 2021 Mar 16. Ann Intern Med. 2021. PMID: 33721521
-
Tenofovir alafenamide (TAF) as the successor of tenofovir disoproxil fumarate (TDF).Biochem Pharmacol. 2016 Nov 1;119:1-7. doi: 10.1016/j.bcp.2016.04.015. Epub 2016 Apr 29. Biochem Pharmacol. 2016. PMID: 27133890 Review.
Cited by
-
Brief communication: comparison of changes in metabolic parameters following antiretrovial therapy with treatment regimens containing tenofovir alafenamide and tenofovir disoproxil fumarate.AIDS Res Ther. 2025 Mar 15;22(1):35. doi: 10.1186/s12981-025-00728-6. AIDS Res Ther. 2025. PMID: 40089788 Free PMC article.
References
-
- Venter WDF, Moorhouse M, Sokhela S, et al. Dolutegravir plus two different prodrugs of tenofovir to treat HIV. N Engl J Med 2019; 381:803–15. - PubMed
-
- Kauppinen KJ, Aho I, Sutinen J. Switching from tenofovir alafenamide to tenofovir disoproxil fumarate improves lipid profile and protects from weight gain. AIDS 2022; 36:1337–44. - PubMed
-
- Mayer KH, Molina JM, Thompson MA, et al. Emtricitabine and tenofovir alafenamide vs emtricitabine and tenofovir disoproxil fumarate for HIV pre-exposure prophylaxis (DISCOVER): primary results from a randomised, double-blind, multicentre, active-controlled, phase 3, non-inferiority trial. Lancet 2020; 396:239–54. - PMC - PubMed
Publication types
MeSH terms
Substances
Associated data
Grants and funding
LinkOut - more resources
Full Text Sources
Medical
Research Materials

