METTL1-modulated LSM14A facilitates proliferation and migration in glioblastoma via the stabilization of DDX5
- PMID: 39040050
- PMCID: PMC11261005
- DOI: 10.1016/j.isci.2024.110225
METTL1-modulated LSM14A facilitates proliferation and migration in glioblastoma via the stabilization of DDX5
Abstract
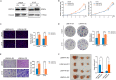
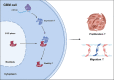
Glioblastoma (GBM) is characterized by aggressive growth, invasiveness, and poor prognosis. Elucidating the molecular mechanisms underlying GBM is crucial. This study explores the role of Sm-like protein 14 homolog A (LSM14A) in GBM. Bioinformatics and clinical tissue samples analysis demonstrated that overexpression of LSM14A in GBM correlates with poorer prognosis. CCK8, EdU, colony formation, and transwell assays revealed that LSM14A promotes proliferation, migration, and invasion in GBM in vitro. In vivo mouse xenograft models confirmed the results of the in vitro experiments. The mechanism of LSM14A modulating GBM cell proliferation was investigated using mass spectrometry, co-immunoprecipitation (coIP), protein half-life, and methylated RNA immunoprecipitation (MeRIP) analyses. The findings indicate that during the G1/S phase, LSM14A stabilizes DDX5 in the cytoplasm, regulating CDK4 and P21 levels. Furthermore, METTL1 modulates LSM14A expression via mRNA m7G methylation. Altogether, our work highlights the METTL1-LSM14A-DDX5 pathway as a potential therapeutic target in GBM.
Keywords: Bioinformatics; Cancer; Molecular biology.
© 2024 The Authors.
Conflict of interest statement
The authors declare no competing interests.
Figures






References
LinkOut - more resources
Full Text Sources

