Macrophage heterogeneity in myocardial infarction: Evolution and implications for diverse therapeutic approaches
- PMID: 39040061
- PMCID: PMC11261154
- DOI: 10.1016/j.isci.2024.110274
Macrophage heterogeneity in myocardial infarction: Evolution and implications for diverse therapeutic approaches
Abstract
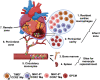
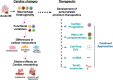
Given the extensive participation of myeloid cells (especially monocytes and macrophages) in both inflammation and resolution phases post-myocardial infarction (MI) owing to their biphasic role, these cells are considered as crucial players in the disease pathogenesis. Multiple studies have agreed on the significant contribution of macrophage polarization theory (M2 vs. M1) while determining the underlying reasons behind the observed biphasic effects; nevertheless, this simplistic classification attracts severe drawbacks. The advent of multiple advanced technologies based on OMICS platforms facilitated a successful path to explore comprehensive cellular signatures that could expedite our understanding of macrophage heterogeneity and plasticity. While providing an overall basis behind the MI disease pathogenesis, this review delves into the literature to discuss the current knowledge on multiple macrophage clusters, including the future directions in this research arena. In the end, our focus will be on outlining the possible therapeutic implications based on the emerging observations.
Keywords: cardiovascular medicine; health sciences; human physiology.
© 2024 The Authors.
Conflict of interest statement
The authors declare no competing interests.
Figures

References
-
- Gerber Y., Weston S.A., Berardi C., McNallan S.M., Jiang R., Redfield M.M., Roger V.L. Contemporary trends in heart failure with reduced and preserved ejection fraction after myocardial infarction: a community study. Am. J. Epidemiol. 2013;178:1272–1280. doi: 10.1093/aje/kwt109. - DOI - PMC - PubMed
Publication types
LinkOut - more resources
Full Text Sources

