From 0D-complex to 3D-MOF: changing the antimicrobial activity of zinc(II) via reaction with aminocinnamic acids
- PMID: 39040090
- PMCID: PMC11260639
- DOI: 10.3389/fchem.2024.1430457
From 0D-complex to 3D-MOF: changing the antimicrobial activity of zinc(II) via reaction with aminocinnamic acids
Abstract
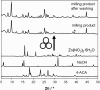
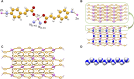
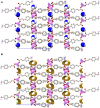
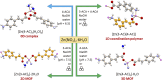
Combining zinc nitrate with 3- and/or 4- aminocinnamic acid (3-ACA and 4-ACA, respectively) leads to the formation of the 0D complex [Zn(4-AC)2(H2O)2], the 1D coordination polymer [Zn(3-AC)(4-AC)], and the 2D and 3D MOFs [Zn(3-AC)2]∙2H2O and [Zn(4-AC)2]∙H2O, respectively. These compounds result from the deprotonation of the acid molecules, with the resulting 3- and 4-aminocinnamate anions serving as bidentate terminal or bridging ligands. All solids were fully characterized via single crystal and powder X-ray diffraction and thermal techniques. Given the mild antimicrobial properties of cinnamic acid derivatives and the antibacterial nature of the metal cation, these compounds were assessed and demonstrated very good planktonic cell killing as well as inhibition of biofilm growth against Pseudomonas aeruginosa, Escherichia coli, and Staphylococcus aureus.
Keywords: MOFs; aminocinnamic acid; antimicrobial activity; mechanochemistry; solid state structure; zinc (II) complexes.
Copyright © 2024 d’Agostino, Macchietti, Turner and Grepioni.
Conflict of interest statement
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Figures



Similar articles
-
Exploring the Co-Crystallization of Kojic Acid with Silver(I), Copper(II), Zinc(II), and Gallium(III) for Potential Antibacterial Applications.Molecules. 2023 Jan 27;28(3):1244. doi: 10.3390/molecules28031244. Molecules. 2023. PMID: 36770910 Free PMC article.
-
A zinc(II)-based two-dimensional MOF for sensitive and selective sensing of HIV-1 ds-DNA sequences.Anal Chim Acta. 2016 May 30;922:55-63. doi: 10.1016/j.aca.2016.03.054. Epub 2016 Apr 7. Anal Chim Acta. 2016. PMID: 27154832
-
Antifungal promising agents of zinc(II) and copper(II) derivatives based on azole drug.J Inorg Biochem. 2021 Jun;219:111401. doi: 10.1016/j.jinorgbio.2021.111401. Epub 2021 Feb 20. J Inorg Biochem. 2021. PMID: 33756392
-
Synthesis, structural characterization and antimicrobial activities of 12 zinc(II) complexes with four thiosemicarbazone and two semicarbazone ligands.J Inorg Biochem. 2003 Aug 1;96(2-3):298-310. doi: 10.1016/s0162-0134(03)00156-9. J Inorg Biochem. 2003. PMID: 12888265
-
A self-penetrated three-dimensional zinc(II) coordination framework based on 4,4',4''-(1,3,5-triazine-2,4,6-triyl)tribenzoic acid and 1,3-bis[(imidazol-1-yl)methyl]benzene ligands: synthesis, structure and properties.Acta Crystallogr C Struct Chem. 2020 Jan 1;76(Pt 1):10-16. doi: 10.1107/S2053229619015547. Epub 2020 Jan 1. Acta Crystallogr C Struct Chem. 2020. PMID: 31919302
References
-
- Alhumaimess M. S. (2020). Metal–organic frameworks and their catalytic applications. J. Saudi Chem. Soc. 24, 461–473. 10.1016/j.jscs.2020.04.002 - DOI
-
- Altomare A., Cuocci C., Giacovazzo C., Moliterni A., Rizzi R., Corriero N., et al. (2013). EXPO2013: a kit of tools for phasing crystal structures from powder data. J. Appl. Crystallogr. 46, 1231–1235. 10.1107/S0021889813013113 - DOI
-
- Altomare A., Giacovazzo C., Guagliardi A., Moliterni A. G. G., Rizzi R., Werner P. E. (2000). New techniques for indexing: N-TREOR in EXPO. J. Appl. Crystallogr. 33, 1180–1186. 10.1107/S0021889800006427 - DOI
LinkOut - more resources
Full Text Sources

