The antipsychotic potential of Salix Mucronata on ketamine-induced rats
- PMID: 39040636
- PMCID: PMC11261032
- DOI: 10.1016/j.ibneur.2024.06.003
The antipsychotic potential of Salix Mucronata on ketamine-induced rats
Abstract
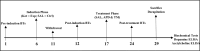
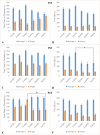
Salix mucronata is one of the herbal plants offered by the traditional health practitioners in KwaZulu-Natal, South Africa for the treatment of schizophrenia. This study aimed to investigate the effects of repeated administration of ketamine on social interaction, novelty and motivation in adult, male Sprague Dawley rats. It also aimed to investigate the potential of risperidone and the herbal extract of S. mucronata to reverse impairments that are induced by ketamine. Experimental rats (n=45) received a dose of ketamine at 30 mg/kg via intraperitoneal injection for 5 consecutive days. They were then allocated into their respective treatment groups and given risperidone (APD) and the herbal extract of S. mucronata (TM) at doses of 6 mg/kg and 5 mg/kg, respectively, for 7 consecutive days. Social behaviour was tested using the 3-chambered sociability test, and anhedonia was tested using the sucrose preference test. Ketamine induction elicited social withdrawal and reduced social novelty which were later successfully reversed by risperidone and S. mucronata. The rats showed reduced preference to sucrose post-induction and post-treatment. Ketamine and mild stress caused by scruff restraint elicited reduced weight gain for the animals. No differences were noted on brain mass between controls and experimental groups and also between risperidone and S. mucronata groups. However, reduced brain volume was noted in experimental groups. Dopamine and acetylcholine concentration levels were high in groups which received risperidone and S. mucronata. These findings highlight that the antipsychotic potential of S. mucronata is similar to risperidone.
Keywords: Herbal medicine; Mental disorders; Risperidone; Salix mucronata; Schizophrenia.
© 2024 The Authors.
Conflict of interest statement
The authors have no conflict of interest to disclose.
Figures







References
-
- de Bartolomeis A., Ciccarelli M., De Simone G., Mazza B., Barone A., Vellucci L. Canonical and non-canonical antipsychotics’ dopamine-related mechanisms of present and next generation molecules: a systematic review on translational highlights for treatment response and treatment-resistant schizophrenia. Int. J. Mol. Sci. 2023;24(6):1–57. doi: 10.3390/ijms24065945. - DOI - PMC - PubMed
LinkOut - more resources
Full Text Sources
