Neoantigen-specific T cell help outperforms non-specific help in multi-antigen DNA vaccination against cancer
- PMID: 39040850
- PMCID: PMC11261851
- DOI: 10.1016/j.omton.2024.200835
Neoantigen-specific T cell help outperforms non-specific help in multi-antigen DNA vaccination against cancer
Abstract
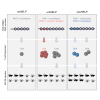
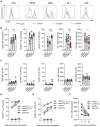
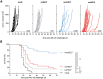
CD4+ T helper antigens are essential components of cancer vaccines, but the relevance of the source of these MHC class II-restricted antigens remains underexplored. To compare the effectiveness of tumor-specific versus tumor-unrelated helper antigens, we designed three DNA vaccines for the murine MC-38 colon carcinoma, encoding CD8+ T cell neoantigens alone (noHELP) or in combination with either "universal" helper antigens (uniHELP) or helper neoantigens (neoHELP). Both types of helped vaccines increased the frequency of vaccine-induced CD8+ T cells, and particularly uniHELP increased the fraction of KLRG1+ and PD-1low effector cells. However, when mice were subsequently injected with MC-38 cells, only neoHELP vaccination resulted in significantly better tumor control than noHELP. In contrast to uniHELP, neoHELP-induced tumor control was dependent on the presence of CD4+ T cells, while both vaccines relied on CD8+ T cells. In line with this, neoHELP variants containing wild-type counterparts of the CD4+ or CD8+ T cell neoantigens displayed reduced tumor control. These data indicate that optimal personalized cancer vaccines should include MHC class II-restricted neoantigens to elicit tumor-specific CD4+ T cell help.
Keywords: DNA vaccine; MT: Regular Issue; cancer vaccine; cytolytic T lymphocytes; helper T lymphocytes; immunotherapy; neoantigens; personalized medicine; synthetic DNA.
© 2024 The Authors.
Conflict of interest statement
B.T. and G.C.Z. are inventors on patent application WO2020218924 – METHODS AND COMPOSITIONS FOR ISOTHERMAL DNA AMPLIFICATION, which describes the technology underlying the production of synthetic, linear DNA vaccines.
Figures



References
-
- Subudhi S.K., Vence L., Zhao H., Blando J., Yadav S.S., Xiong Q., Reuben A., Aparicio A., Corn P.G., Chapin B.F., et al. Neoantigen responses, immune correlates, and favorable outcomes after ipilimumab treatment of patients with prostate cancer. Sci. Transl. Med. 2020;12 - PubMed
-
- Chesney J., Awasthi S., Curti B., Hutchins L., Linette G., Triozzi P., Tan M.C.B., Brown R.E., Nemunaitis J., Whitman E., et al. Phase IIIb safety results from an expanded-access protocol of talimogene laherparepvec for patients with unresected, stage IIIB-IVM1c melanoma. Melanoma Res. 2018;28:44–51. - PubMed
-
- Carreno B.M., Magrini V., Becker-Hapak M., Kaabinejadian S., Hundal J., Petti A.A., Ly A., Lie W.-R., Hildebrand W.H., Mardis E.R., Linette G.P. Cancer immunotherapy. A dendritic cell vaccine increases the breadth and diversity of melanoma neoantigen-specific T cells. Science. 2015;348:803–808. - PMC - PubMed
LinkOut - more resources
Full Text Sources
Research Materials
