Bioanalysis of free antisense oligonucleotide payload from antibody-oligonucleotide conjugate by hybridization LC-MS/MS
- PMID: 39041663
- PMCID: PMC11415018
- DOI: 10.1080/17576180.2024.2368339
Bioanalysis of free antisense oligonucleotide payload from antibody-oligonucleotide conjugate by hybridization LC-MS/MS
Abstract
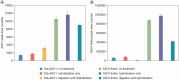
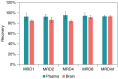
Background: Antisense oligonucleotides (ASOs) have been conjugated to various moieties, such as peptides, antibodies or Fab regions of antibodies, to enhance their delivery to target tissues. The quantitation of free ASO (ASO payload) is critical to characterize its pharmacokinetics/pharmacodynamics (PK/PD) properties and biodistribution after delivery of the peptide/antibody/Fab ASO conjugates.Results: We developed a hybridization-based LC-MS/MS methodology for quantification of free ASO in tissues in the presence of Fab-ASO and ASO with linker (ASO-linker).Conclusion: The developed method was applied to measure accurately the free ASO concentrations in liver and gastrocnemius in mice that were dosed with Fab-ASO. This methodology has also been applied to free ASO bioanalysis for other antibody-ASO and Fab-ASO conjugates in various tissues and plasma/serum samples.
Keywords: Fab–ASO conjugates; LC-MS/MS; antibody–ASO conjugates; antibody–oligonucleotide conjugates (AOCs); antisense oligonucleotides (ASOs); bioanalysis; hybridization.
Plain language summary
[Box: see text].
Conflict of interest statement
The authors have no competing interests or relevant affiliations with any organization or entity with the subject matter or materials discussed in the manuscript. This includes employment, consultancies, stock ownership or options and expert testimony.
Figures


Similar articles
-
Validation and application of hybridization liquid chromatography-tandem mass spectrometry methods for quantitative bioanalysis of antisense oligonucleotides.Bioanalysis. 2022 May;14(9):589-601. doi: 10.4155/bio-2022-0015. Epub 2022 May 12. Bioanalysis. 2022. PMID: 35545949
-
Hybridization Liquid Chromatography-Tandem Mass Spectrometry: An Alternative Bioanalytical Method for Antisense Oligonucleotide Quantitation in Plasma and Tissue Samples.Anal Chem. 2020 Aug 4;92(15):10548-10559. doi: 10.1021/acs.analchem.0c01382. Epub 2020 Jul 17. Anal Chem. 2020. PMID: 32628461
-
Microflow LC-MS/MS to improve sensitivity for antisense oligonucleotides bioanalysis: critical role of sample cleanness.Bioanalysis. 2022 Nov;14(21):1365-1376. doi: 10.4155/bio-2022-0201. Epub 2023 Jan 10. Bioanalysis. 2022. PMID: 36625771
-
Distribution and biotransformation of therapeutic antisense oligonucleotides and conjugates.Drug Discov Today. 2021 Oct;26(10):2244-2258. doi: 10.1016/j.drudis.2021.04.002. Epub 2021 Apr 20. Drug Discov Today. 2021. PMID: 33862193 Review.
-
Bioanalytical approaches to support the development of antibody-oligonucleotide conjugate (AOC) therapeutic proteins.Xenobiotica. 2024 Aug;54(8):552-562. doi: 10.1080/00498254.2024.2339983. Epub 2024 Sep 27. Xenobiotica. 2024. PMID: 38607350 Review.
Cited by
-
Regulated bioanalysis of antibody-drug conjugates using LC-MS.Bioanalysis. 2025 Apr;17(8):549-560. doi: 10.1080/17576180.2025.2490468. Epub 2025 Apr 9. Bioanalysis. 2025. PMID: 40205765 Review.
-
Quantitative Determination of Click-Reactive Antisense Oligonucleotide and Its Reactivity in Rat Brains by Hybridization LC-MS/MS to Support Pretargeted PET Imaging.Anal Chem. 2025 Jun 17;97(23):12155-12163. doi: 10.1021/acs.analchem.5c00644. Epub 2025 Jun 4. Anal Chem. 2025. PMID: 40464260 Free PMC article.
References
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
