Decitabine enclosed biotin-zein conjugated nanoparticles: synthesis, characterization, in vitro and in vivo evaluation
- PMID: 39041671
- PMCID: PMC11418219
- DOI: 10.1080/17435889.2024.2374700
Decitabine enclosed biotin-zein conjugated nanoparticles: synthesis, characterization, in vitro and in vivo evaluation
Abstract
Aim: This study focuses on biotinylated nanocarriers designed to encapsulate amphiphilic molecules with self-biodegradable properties for enhanced drug delivery.Methods: Biotin-zein conjugated nanoparticles were synthesized and tested in C6 cell lines to evaluate their viability and cellular uptake. Optimization was achieved using a a central composite design. The nanoparticles underwent thermogravimetric analysis, and their pharmacokinetics and biodistribution were also studied.Results: The optimized nanoparticles displayed 96.31% drug encapsulation efficiency, a particle size of 95.29 nm and a zeta potential of -17.7 mV. These nanoparticles showed increased cytotoxicity and improved cellular uptake compared with free drugs. Thermogravimetric analysis revealed that the drug-loaded nanocarriers provided better protection against drug degradation. Pharmacokinetic and biodistribution studies indicated that the formulation had an extended brain residence time, highlighting its effectiveness.Conclusion: The biotin-zein conjugated nanoparticles developed in this study offer a promising nano-vehicle for in vivo biodistribution and pharmacokinetic applications. Their high drug encapsulation efficiency, stability and extended brain residence time suggest they are effective for targeted drug delivery and therapeutic uses.
Keywords: C6 cell line; biodistribution; central composite design; decitabine; in vivo bioimaging; nanoparticle; zein.
Plain language summary
[Box: see text].
Conflict of interest statement
The authors have no competing interests or relevant affiliations with any organization or entity with the subject matter or materials discussed in the manuscript. This includes employment, consultancies, honoraria, stock ownership or options, expert testimony, grants or patents received or pending, or royalties.
Figures


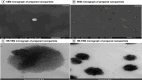

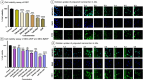

References
-
- Gomez-Estaca J, Balaguer MP, Gavara R, et al. Formation of zein nanoparticles by electrohydrodynamic atomization: effect of the main processing variables and suitability for encapsulating the food coloring and active ingredient curcumin. Food Hydrocoll. 2012;28:82–91. doi: 10.1016/j.foodhyd.2011.11.013 - DOI
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
