A mechanistic PK/PD model of AZD0171 (anti-LIF) to support Phase II dose selection
- PMID: 39041713
- PMCID: PMC11494920
- DOI: 10.1002/psp4.13204
A mechanistic PK/PD model of AZD0171 (anti-LIF) to support Phase II dose selection
Abstract
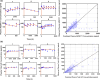
AZD0171 (INN: Falbikitug) is being developed as a humanized monoclonal antibody (mAb), immunoglobulin G subclass 1 (IgG1), which binds specifically to the immunosuppressive human cytokine leukemia inhibitory factor (LIF) and inhibits downstream signaling by blocking recruitment of glycoprotein 130 (gp130) to the LIF receptor (LIFR) subunit (gp190) and the phosphorylation of signal transducer and activator of transcription 3 (STAT3) and is intended to treat adult participants with advanced solid tumors. LIF is a pleiotropic cytokine (and a member of the IL-6 family of cytokines) involved in many physiological and pathological processes and is highly expressed in a subset of solid tumors, including non-small cell lung cancer (NSCLC), colon, ovarian, prostate, and pancreatic cancer. The aim of this work was to develop a mechanistic PK/PD model to investigate the effect of AZD0171 on tumor LIF levels, predict the level of downstream signaling complex (LIF:LIFR:gp130) inhibition, and examine the dose-response relationship to support dose selection for a Phase II clinical study. Modeling results show that tumor LIF is inhibited in a dose-dependent manner with >90% inhibition for 95% of patients at the Phase II clinical dose of 1500 mg Q2W.
© 2024 The Author(s). CPT: Pharmacometrics & Systems Pharmacology published by Wiley Periodicals LLC on behalf of American Society for Clinical Pharmacology and Therapeutics.
Conflict of interest statement
A.S., M.P., J.C., G.A., M.N., J.E., O.O., N.L, N.M.L., A.P., and K.V. are employees and stockholders of AstraZeneca pharmaceuticals.
Figures



References
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Miscellaneous

