Whence and wherefore IgE?
- PMID: 39041740
- PMCID: PMC11436312
- DOI: 10.1111/imr.13373
Whence and wherefore IgE?
Abstract
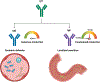
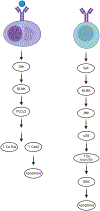
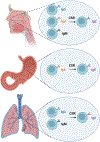
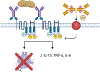
Despite the near ubiquitous presence of Ig-based antibodies in vertebrates, IgE is unique to mammals. How and why it emerged remains mysterious. IgE expression is greatly constrained compared to other IgH isotypes. While other IgH isotypes are relatively abundant, soluble IgE has a truncated half-life, and IgE plasma cells are mostly short-lived. Despite its rarity, IgE is consequential and can trigger life-threatening anaphylaxis. IgE production reflects a dynamic steady state with IgG memory B cells feeding short-lived IgE production. Emerging evidence suggests that IgE may also potentially be produced in longer-lived plasma cells as well, perhaps as an aberrancy stemming from its evolutionary roots from an antibody isotype that likely functioned more like IgG. As a late derivative of an ancient systemic antibody system, the benefits of IgE in mammals likely stems from the antibody system's adaptive recognition and response capability. However, the tendency for massive, systemic, and long-lived production, common to IgH isotypes like IgG, were likely not a good fit for IgE. The evolutionary derivation of IgE from an antibody system that for millions of years was good at antigen de-sensitization to now functioning as a highly specialized antigen-sensitization function required heavy restrictions on antibody production-insufficiency of which may contribute to allergic disease.
Keywords: IgE; IgG; allergen immunotherapy; allergy; class switch recombination; plasma cells.
© 2024 John Wiley & Sons A/S. Published by John Wiley & Sons Ltd.
Conflict of interest statement
Conflicts of Interest Statement
Authors report no relevant conflicts.
Figures



References
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Research Materials

