Impact of Discontinuing Both Hypertonic Saline and Dornase Alfa after Elexacaftor-Tezacaftor-Ivacaftor in Cystic Fibrosis
- PMID: 39041864
- PMCID: PMC11568493
- DOI: 10.1513/AnnalsATS.202404-366OC
Impact of Discontinuing Both Hypertonic Saline and Dornase Alfa after Elexacaftor-Tezacaftor-Ivacaftor in Cystic Fibrosis
Abstract
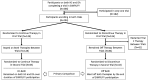
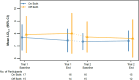
Rationale: Evaluating approaches to reduce treatment burden is a research priority among people with cystic fibrosis on highly effective modulators, including elexacaftor-tezacaftor-ivacaftor (ETI). Objectives: We sought to evaluate the impact of discontinuing both hypertonic saline (HS) and dornase alfa (DA) versus continuing both therapies among a subgroup of participants in the SIMPLIFY study who sequentially participated in trials evaluating the independent clinical effects of discontinuing HS and DA. Methods: SIMPLIFY participants ≥12 years old on ETI and constituting a subgroup using both HS and DA at study entry were randomized to the HS or DA trial and then randomized 1:1 to continue or discontinue the applicable therapy for 6 weeks. After completion of the first trial, eligible participants could enroll in the second trial beginning with a 2-week run-in. Study outcomes were compared across the duration of SIMPLIFY participation between a cohort remaining on both therapies during SIMPLIFY and a cohort that sequentially discontinued both as a result of trial randomizations. Multivariable regression models were used to estimate treatment differences, adjusted for time between trials, trial order, baseline age, sex at birth, and percent predicted forced expiratory volume in 1 second (ppFEV1) at study entry. Results: Forty-three participants discontinued both therapies by the end of SIMPLIFY, and 63 remained on both, with overall average ppFEV1 of 96.7% at study entry and 3.9 months as the average duration of follow-up from beginning of the first trial to completion of the second trial, including time between trials. No clinically meaningful difference in the change in ppFEV1 from baseline to completion of the second trial was observed between those who discontinued and those who remained on both therapies (difference: 0.22% off-on; 95% confidence interval = -1.60, 2.03). Changes in lung clearance index at 2.5% starting concentration, patient-reported outcomes, and safety outcomes were also comparable. Patient-reported treatment burden, as measured by a Cystic Fibrosis Questionnaire-Revised subscale, significantly decreased in those who discontinued both therapies. Conclusions: SIMPLIFY participants who sequentially discontinued both HS and DA experienced no meaningful changes in clinical outcomes and reported decreased treatment burden as compared with those who remained on both therapies. These data continue to inform a new era of postmodulator care of people with cystic fibrosis.
Keywords: CFTR modulators; treatment burden; treatment discontinuation.
Figures


Comment in
-
Reducing Treatment Burden in Cystic Fibrosis: Is There a Risk to OverSIMPLIFYing Therapies?Ann Am Thorac Soc. 2024 Nov;21(11):1483-1484. doi: 10.1513/AnnalsATS.202407-694ED. Ann Am Thorac Soc. 2024. PMID: 39485168 Free PMC article. No abstract available.
References
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical

