Clinical and Genomic Risk for Late Breast Cancer Recurrence and Survival
- PMID: 39041867
- PMCID: PMC11730053
- DOI: 10.1056/EVIDoa2300267
Clinical and Genomic Risk for Late Breast Cancer Recurrence and Survival
Abstract
Background: The 21-gene recurrence score (RS) assay (Oncotype DX) is used to guide adjuvant chemotherapy use for patients with hormone receptor-positive, HER2 (human epidermal growth factor receptor 2)-negative, axillary node-negative breast cancer. Its role, however, in providing prognostic information for late distant recurrence when added to clinicopathologic prognostic factors is unknown.
Methods: A patient-specific meta-analysis including 10,004 women enrolled in three trials was updated using extended follow-up data from TAILORx, integrating the RS with histologic grade, tumor size, and age at surgery for the RSClin tool. Cox models integrating clinicopathologic factors and the RS were compared by using likelihood ratio (LR) tests. External validation of prognosis for distant recurrence in years 0 to 10 and 5 to 10 was performed in an independent cohort of 1098 women in a real-world registry.
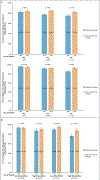
Results: RSClin provided significantly more prognostic information than either the clinicopathologic factors (ΔLR chi-square, 86.2; P<0.001) or RS alone (ΔLR chi-square, 131.0; P<0.001). The model was prognostic in an independent cohort for distant recurrence by 10 years after diagnosis (standardized hazard ratio, 1.56; 95% confidence interval, 1.25 to 1.94), was associated with late distant recurrence risk between 5 and 10 years after diagnosis (standardized hazard ratio, 1.78; 95% confidence interval, 1.25 to 2.55), and approximated the observed 10-year distant recurrence risk (Lin concordance, 0.87) and 5- to 10-year distant recurrence risk (Lin concordance, 0.92).
Conclusions: The 21-gene RS is prognostic for distant recurrence and overall survival in early breast cancer. A model integrating the 21-gene RS and clinicopathologic factors improved estimates of distant recurrence risk compared with either used individually and stratified late distant recurrence risk. (Funded by the National Cancer Institute, National Institutes of Health [U10CA180820, U10CA180794, UG1CA189859, U10CA180868, and U10CA180822] and others.).
Figures


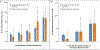
References
Publication types
MeSH terms
Grants and funding
LinkOut - more resources
Full Text Sources
Medical
Research Materials
Miscellaneous
