Preliminary Findings From the Dynamics of the Immune Responses to Repeat Influenza Vaccination Exposures (DRIVE I) Study: A Randomized Controlled Trial
- PMID: 39041887
- PMCID: PMC11478574
- DOI: 10.1093/cid/ciae380
Preliminary Findings From the Dynamics of the Immune Responses to Repeat Influenza Vaccination Exposures (DRIVE I) Study: A Randomized Controlled Trial
Abstract
Background: Studies have reported that repeated annual vaccination may influence influenza vaccination effectiveness in the current season.
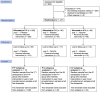
Methods: We established a 5-year randomized placebo-controlled trial of repeated influenza vaccination (Flublok; Sanofi Pasteur) in adults 18-45 years of age. In the first 2 years, participants were randomized to receive vaccine or saline placebo as follows: placebo-placebo (P-P), placebo-vaccine (P-V), or vaccine-vaccine (V-V). Serum samples were collected each year just before vaccination and after 30 and 182 days. A subset of serum samples collected at 5 time points from 95 participants were tested for antibodies against vaccine strains.
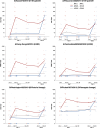
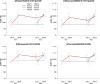
Results: From 23 October 2020 through 11 March 2021 we enrolled and randomized 447 adults. Among vaccinated individuals, antibody titers increased between days 0 and 30 against each of the vaccine strains, with smaller increases for repeat vaccinees who on average had higher prevaccination titers in year 2. There were statistically significant differences in the proportions of participants achieving ≥4-fold rises in antibody titer for the repeat vaccinees for influenza A(H1N1), B/Victoria, and B/Yamagata, but not for A(H3N2). Among participants who received vaccination in year 2, there were no significant differences between the P-V and V-V groups in geometric mean titers at day 30 or the proportions of participants with antibody titers ≥40 at day 30 for any of the vaccine strains.
Conclusions: In the first 2 years, during which influenza did not circulate, repeat and first-time vaccinees had similar postvaccination geometric mean titers to all 4 vaccine strains, indicative of similar levels of clinical protection. Clinical Trials Registration. NCT04576377.
Keywords: ELISA; antibody; immunogenicity; influenza; vaccination.
© The Author(s) 2024. Published by Oxford University Press on behalf of Infectious Diseases Society of America. All rights reserved. For commercial re-use, please contact reprints@oup.com for reprints and translation rights for reprints. All other permissions can be obtained through our RightsLink service via the Permissions link on the article page on our site—for further information please contact journals.permissions@oup.com.
Conflict of interest statement
Potential conflicts of interest . B. J. C. consults for AstraZeneca, Fosun Pharma, GlaxoSmithKline, Haleon, Moderna, Novavax, Pfizer, Roche, and Sanofi Pasteur. S. E. H. is a coinventor on patents that describe the use of nucleoside-modified messenger RNA as a vaccine platform; S. E. H. also reports receiving consulting fees from Sanofi, Pfizer, Lumen, Novavax, and Merck. S. C. has consulted for Seqirus. All other authors report no potential conflicts. All authors have submitted the ICMJE Form for Disclosure of Potential Conflicts of Interest. Conflicts that the editors consider relevant to the content of the manuscript have been disclosed.
Figures
Update of
-
Preliminary findings from the Dynamics of the Immune Responses to Repeat Influenza Vaccination Exposures (DRIVE I) Study: a Randomized Controlled Trial.medRxiv [Preprint]. 2024 May 17:2024.05.16.24307455. doi: 10.1101/2024.05.16.24307455. medRxiv. 2024. Update in: Clin Infect Dis. 2024 Oct 15;79(4):901-909. doi: 10.1093/cid/ciae380. PMID: 38798684 Free PMC article. Updated. Preprint.
References
-
- Belongia EA, Skowronski DM, McLean HQ, Chambers C, Sundaram ME, De Serres G. Repeated annual influenza vaccination and vaccine effectiveness: review of evidence. Expert Rev Vaccines 2017; 16:723–36. - PubMed
Publication types
MeSH terms
Substances
Associated data
Grants and funding
LinkOut - more resources
Full Text Sources
Medical
Miscellaneous

