Minimal Functionalization of Ruthenium Compounds with Enhanced Photoreactivity against Hard-to-Treat Cancer Cells and Resistant Bacteria
- PMID: 39042379
- PMCID: PMC11304396
- DOI: 10.1021/acs.inorgchem.4c02235
Minimal Functionalization of Ruthenium Compounds with Enhanced Photoreactivity against Hard-to-Treat Cancer Cells and Resistant Bacteria
Abstract
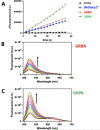
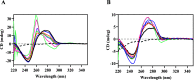
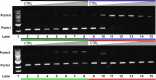
Metallocompounds have emerged as promising new anticancer agents, which can also exhibit properties to be used in photodynamic therapy. Here, we prepared two ruthenium-based compounds with a 2,2'-bipyridine ligand conjugated to an anthracenyl moiety. These compounds coded GRBA and GRPA contain 2,2'-bipyridine or 1,10-phenathroline as auxiliary ligands, respectively, which provide quite a distinct behavior. Notably, compound GRPA exhibited remarkably high photoproduction of singlet oxygen even in water (ϕΔ = 0.96), almost twice that of GRBA (ϕΔ = 0.52). On the other hand, this latter produced twice more superoxide and hydroxyl radical species than GRPA, which may be due to the modulation of their excited state. Interestingly, GRPA exhibited a modest binding to DNA (Kb = 4.51 × 104), while GRBA did not show a measurable interaction only noticed by circular dichroism measurements. Studies with bacteria showed a great antimicrobial effect, including a synergistic effect in combination with commercial antibiotics. Besides that, GRBA showed very low or no cytotoxicity against four mammalian cells, including a hard-to-treat MDA-MB-231, triple-negative human breast cancer. Potent activities were measured for GRBA upon blue light irradiation, where IC50 of 43 and 13 nmol L-1 were seen against hard-to-treat triple-negative human breast cancer (MDA-MB-231) and ovarian cancer cells (A2780), respectively. These promising results are an interesting case of a simple modification with expressive enhancement of biological activity that deserves further biological studies.
Conflict of interest statement
The authors declare no competing financial interest.
Figures







References
-
- Monro S.; Colón K. L.; Yin H.; Roque J.; Konda P.; Gujar S.; Thummel R. P.; Lilge L.; Cameron C. G.; McFarland S. A. Transition Metal Complexes and Photodynamic Therapy from a Tumor-Centered Approach: Challenges, Opportunities, and Highlights from the Development of TLD1433. Chem. Rev. 2019, 119 (2), 797–828. 10.1021/acs.chemrev.8b00211. - DOI - PMC - PubMed
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Medical
Molecular Biology Databases
Miscellaneous

