Enteric neural stem cell transplant restores gut motility in mice with Hirschsprung disease
- PMID: 39042470
- PMCID: PMC11385093
- DOI: 10.1172/jci.insight.179755
Enteric neural stem cell transplant restores gut motility in mice with Hirschsprung disease
Abstract
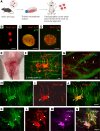
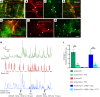
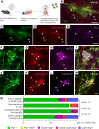
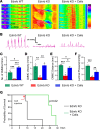
The goal of this study was to determine if transplantation of enteric neural stem cells (ENSCs) can rescue the enteric nervous system, restore gut motility, reduce colonic inflammation, and improve survival in the Ednrb-KO mouse model of Hirschsprung disease (HSCR). ENSCs were isolated from mouse intestine, expanded to form neurospheres, and microinjected into the colons of recipient Ednrb-KO mice. Transplanted ENSCs were identified in recipient colons as cell clusters in "neo-ganglia." Immunohistochemical evaluation demonstrated extensive cell migration away from the sites of cell delivery and across the muscle layers. Electrical field stimulation and optogenetics showed significantly enhanced contractile activity of aganglionic colonic smooth muscle following ENSC transplantation and confirmed functional neuromuscular integration of the transplanted ENSC-derived neurons. ENSC injection also partially restored the colonic migrating motor complex. Histological examination revealed a significant reduction in inflammation in ENSC-transplanted aganglionic recipient colon compared with that of sham-operated mice. Interestingly, mice that received cell transplant also had prolonged survival compared with controls. This study demonstrates that ENSC transplantation can improve outcomes in HSCR by restoring gut motility and reducing the severity of Hirschsprung-associated enterocolitis, the leading cause of death in human HSCR.
Keywords: Cell biology; Gastroenterology; Neurodevelopment; Neuronal stem cells; Stem cell transplantation.
Figures


References
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Molecular Biology Databases
Research Materials

