Systemic and skin-limited delayed-type drug hypersensitivity reactions associate with distinct resident and recruited T cell subsets
- PMID: 39042477
- PMCID: PMC11364394
- DOI: 10.1172/JCI178253
Systemic and skin-limited delayed-type drug hypersensitivity reactions associate with distinct resident and recruited T cell subsets
Abstract
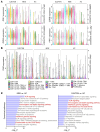
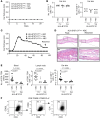
Delayed-type drug hypersensitivity reactions are major causes of morbidity and mortality. The origin, phenotype, and function of pathogenic T cells across the spectrum of severity require investigation. We leveraged recent technical advancements to study skin-resident memory T cells (TRMs) versus recruited T cell subsets in the pathogenesis of severe systemic forms of disease, Stevens-Johnson syndrome/toxic epidermal necrolysis (SJS/TEN) and drug reaction with eosinophilia and systemic symptoms (DRESS), and skin-limited disease, morbilliform drug eruption (MDE). Microscopy, bulk transcriptional profiling, and single-cell RNA-sequencing (scRNA-Seq) plus cellular indexing of transcriptomes and epitopes by sequencing (CITE-Seq) plus T cell receptor sequencing (TCR-Seq) supported clonal expansion and recruitment of cytotoxic CD8+ T cells from circulation into skin along with expanded and nonexpanded cytotoxic CD8+ skin TRM in SJS/TEN. Comparatively, MDE displayed a cytotoxic T cell profile in skin without appreciable expansion and recruitment of cytotoxic CD8+ T cells from circulation, implicating TRMs as potential protagonists in skin-limited disease. Mechanistic interrogation in patients unable to recruit T cells from circulation into skin and in a parallel mouse model supported that skin TRMs were sufficient to mediate MDE. Concomitantly, SJS/TEN displayed a reduced Treg signature compared with MDE. DRESS demonstrated recruitment of cytotoxic CD8+ T cells into skin as in SJS/TEN, yet a pro-Treg signature as in MDE. These findings have important implications for fundamental skin immunology and clinical care.
Keywords: Allergy; Dermatology; Immunology; Skin; T cells.
Figures





References
MeSH terms
Grants and funding
LinkOut - more resources
Full Text Sources
Molecular Biology Databases
Research Materials

