N-Glycosylation-Induced Pathologic Protein Conformations as a Tool to Guide the Selection of Biologically Active Small Molecules
- PMID: 39042517
- PMCID: PMC12000882
- DOI: 10.1002/chem.202401957
N-Glycosylation-Induced Pathologic Protein Conformations as a Tool to Guide the Selection of Biologically Active Small Molecules
Abstract
Post-translational modifications such as protein N-glycosylation, significantly influence cellular processes. Dysregulated N-glycosylation, exemplified in Grp94, a member of the Hsp90 family, leads to structural changes and the formation of epichaperomes, contributing to pathologies. Targeting N-glycosylation-induced conformations offers opportunities for developing selective chemical tools and drugs for these pathologic forms of chaperones. We here demonstrate how a specific Grp94 conformation induced by N-glycosylation, identified previously via molecular dynamics simulations, rationalizes the distinct behavior of similar ligands. Integrating dynamic ligand unbinding information with SAR development, we differentiate ligands productively engaging the pathologic Grp94 conformers from those that are not. Additionally, analyzing binding site stereoelectronic properties and QSAR models using cytotoxicity data unveils relationships between chemical, conformational properties, and biological activities. These findings facilitate the design of ligands targeting specific Grp94 conformations induced by abnormal glycosylation, selectively disrupting pathogenic protein networks while sparing normal mechanisms.
Keywords: Chaperones; Drug design; Molecular dynamics; Post-translational modifications.
© 2024 The Author(s). Chemistry - A European Journal published by Wiley-VCH GmbH.
Conflict of interest statement
Conflict of Interests
G.C., S. D.G., S.O., A.R., H. J.P. and S.S. are inventors on patent applications related to the epichaperome portfolio. All other authors declare no conflicts of interest.
Figures

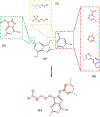

References
-
- Wu BX, Hong F, Zhang Y, Ansa-Addo E, Li Z, Adv. Cancer Res. 2016, 129, 165–190. - PubMed
MeSH terms
Substances
Grants and funding
- R56 AG061869/AG/NIA NIH HHS/United States
- R21 CA158609/CA/NCI NIH HHS/United States
- R56 AG072599/AG/NIA NIH HHS/United States
- R01 AG072599/AG/NIA NIH HHS/United States
- Programma di ricerca CN00000013/Ministero dell'Università e della Ricerca
- U01 AG032969/AG/NIA NIH HHS/United States
- R01 CA172546/CA/NCI NIH HHS/United States
- IMMUNO-HUB, T4-CN-02,/Ministero della Salute
- R01 AG085572/AG/NIA NIH HHS/United States
- R01 AG067598/AG/NIA NIH HHS/United States
- R21 AI090501/AI/NIAID NIH HHS/United States
- RF1 AG071805/AG/NIA NIH HHS/United States
- IG 2022 - ID. 27139/Fondazione AIRC per la ricerca sul cancro ETS
- P30 CA008748/CA/NCI NIH HHS/United States
- P01 CA186866/CA/NCI NIH HHS/United States
- R01 AG074004/AG/NIA NIH HHS/United States
- R01 CA172546, P01 CA186866, R56 AG061869, RF1 AG071805, R01 AG067598, R01 AG074004, R01 AG072599, P30 CA08748/GF/NIH HHS/United States
LinkOut - more resources
Full Text Sources
Miscellaneous

