Generation of antitumor chimeric antigen receptors incorporating T cell signaling motifs
- PMID: 39042728
- PMCID: PMC11389647
- DOI: 10.1126/scisignal.adp8569
Generation of antitumor chimeric antigen receptors incorporating T cell signaling motifs
Abstract
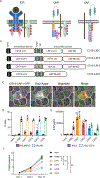
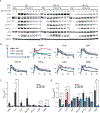
Chimeric antigen receptor (CAR) T cells have been used to successfully treat various blood cancers, but adverse effects have limited their potential. Here, we developed chimeric adaptor proteins (CAPs) and CAR tyrosine kinases (CAR-TKs) in which the intracellular ζ T cell receptor (TCRζ) chain was replaced with intracellular protein domains to stimulate signaling downstream of the TCRζ chain. CAPs contain adaptor domains and the kinase domain of ZAP70, whereas CAR-TKs contain only ZAP70 domains. We hypothesized that CAPs and CAR-TKs would be more potent than CARs because they would bypass both the steps that define the signaling threshold of TCRζ and the inhibitory regulation of upstream molecules. CAPs were too potent and exhibited high tonic signaling in vitro. In contrast, CAR-TKs exhibited high antitumor efficacy and significantly enhanced long-term tumor clearance in leukemia-bearing NSG mice as compared with the conventional CD19-28ζ-CAR-T cells. CAR-TKs were activated in a manner independent of the kinase Lck and displayed slower phosphorylation kinetics and prolonged signaling compared with the 28ζ-CAR. Lck inhibition attenuated CAR-TK cell exhaustion and improved long-term function. The distinct signaling properties of CAR-TKs may therefore be harnessed to improve the in vivo efficacy of T cells engineered to express an antitumor chimeric receptor.
Conflict of interest statement
Figures





References
-
- Kochenderfer JN, Dudley ME, Feldman SA, Wilson WH, Spaner DE, Maric I, Stetler-Stevenson M, Phan GQ, Hughes MS, Sherry RM, Yang JC, Kammula US, Devillier L, Carpenter R, Nathan DA, Morgan RA, Laurencot C, Rosenberg SA, B-cell depletion and remissions of malignancy along with cytokine-associated toxicity in a clinical trial of anti-CD19 chimeric-antigen-receptor-transduced T cells. Blood 119, 2709–2720 (2012); published online EpubMar 22 (10.1182/blood-2011-10-384388). - DOI - PMC - PubMed
-
- Kochenderfer JN, Wilson WH, Janik JE, Dudley ME, Stetler-Stevenson M, Feldman SA, Maric I, Raffeld M, Nathan DA, Lanier BJ, Morgan RA, Rosenberg SA, Eradication of B-lineage cells and regression of lymphoma in a patient treated with autologous T cells genetically engineered to recognize CD19. Blood 116, 4099–4102 (2010); published online EpubNov 18 (10.1182/blood-2010-04-281931). - DOI - PMC - PubMed
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Molecular Biology Databases
Research Materials
Miscellaneous

