Pretransplant Screening for Prevention of Hyperacute Graft Loss in Pig-to-primate Kidney Xenotransplantation
- PMID: 39042769
- PMCID: PMC11287055
- DOI: 10.1097/TP.0000000000004958
Pretransplant Screening for Prevention of Hyperacute Graft Loss in Pig-to-primate Kidney Xenotransplantation
Abstract
Background: Xenotransplantation using pig organs is now a clinical reality. However, the process for xenograft recipient screening lacks clarity and scientific rigor: no established thresholds exist to determine which levels of preformed antipig natural antibodies (Nabs) will be safe for clinical xenograft transplantation, and hyperacute rejection (HAR) or acute humoral xenograft rejection (AHXR), which still impacts pig-to-primate kidney xenograft survivals, may impede broader application of pig-to-human clinical xenograft transplantation.
Methods: We retrospectively examined 28 cases of pig-to-baboon kidney xenotransplantation using GalTKO±human complement regulatory protein (hCRP)-transgenic (Tg) pig donors, as well as 6 cases of triple-KO multi-Tg (10GE) pig donors, and developed screening algorithms to predict risk of HAR/AHXR based on recipient antipig Nab levels. Preformed Nabs were evaluated using both complement-dependent cytotoxicity and antibody (IgM and IgG) binding flow-cytometry assays.
Results: High complement-dependent cytotoxicity was associated with HAR/AHXR as expected. However, we also found that high levels of IgG were independently associated with HAR/AHXR, and we developed 2 indices to interpret and predict the risk of IgG-mediated HAR/AHXR.
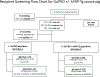
Conclusions: Based on the data in this study, we have established a new 2-step screening, which will be used for future clinical kidney xenotransplantation trials.
Copyright © 2024 Wolters Kluwer Health, Inc. All rights reserved.
Conflict of interest statement
The authors declare no conflicts of interest.
Figures




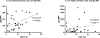

Comment in
-
Reducing Early Graft Loss in Xenotransplantation With Histocompatibility Testing.Transplantation. 2024 Aug 1;108(8):1687-1688. doi: 10.1097/TP.0000000000005020. Epub 2024 Apr 5. Transplantation. 2024. PMID: 38578706 Free PMC article. No abstract available.
References
-
- OPTN: Organ Procurement and Transplantation Network. https://optn.transplant.hrsa.gov/data/
-
- Center for Biologics E, Research. Guidance for industry : public health issues posed by the use of nonhuman primate xenografts in humans. U.S. Dept. of Health and Human Services, Food and Drug Administration, Center for Biologics Evaluation and Research; 1999.
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical
Research Materials
Miscellaneous

