Endothelial RUNX3 controls LSEC dysfunction and angiocrine LRG1 signaling to prevent liver fibrosis
- PMID: 39042837
- PMCID: PMC11902585
- DOI: 10.1097/HEP.0000000000001018
Endothelial RUNX3 controls LSEC dysfunction and angiocrine LRG1 signaling to prevent liver fibrosis
Abstract
Background and aims: Liver fibrosis represents a global health burden, given the paucity of approved antifibrotic therapies. Liver sinusoidal endothelial cells (LSECs) play a major gatekeeping role in hepatic homeostasis and liver disease pathophysiology. In early tumorigenesis, runt-related transcription factor 3 (RUNX3) functions as a sentinel; however, its function in liver fibrosis in LSECs remains unclear. This study aimed to investigate the role of RUNX3 as an important regulator of the gatekeeping functions of LSECs and explore novel angiocrine regulators of liver fibrosis.
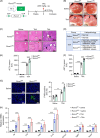
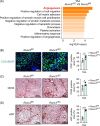
Approach and results: Mice with endothelial Runx3 deficiency develop gradual and spontaneous liver fibrosis secondary to LSEC dysfunction, thereby more prone to liver injury. Mechanistic studies in human immortalized LSECs and mouse primary LSECs revealed that IL-6/JAK/STAT3 pathway activation was associated with LSEC dysfunction in the absence of RUNX3. Single-cell RNA sequencing and quantitative RT-PCR revealed that leucine-rich alpha-2-glycoprotein 1 ( LRG1 ) was highly expressed in RUNX3-deficient and dysfunctional LSECs. In in vitro and coculture experiments, RUNX3-depleted LSECs secreted LRG1, which activated HSCs throughTGFBR1-SMAD2/3 signaling in a paracrine manner. Furthermore, circulating LRG1 levels were elevated in mouse models of liver fibrosis and in patients with fatty liver and cirrhosis.
Conclusions: RUNX3 deficiency in the endothelium induces LSEC dysfunction, LRG1 secretion, and liver fibrosis progression. Therefore, endothelial RUNX3 is a crucial gatekeeping factor in LSECs, and profibrotic angiocrine LRG1 may be a novel target for combating liver fibrosis.
Copyright © 2024 The Author(s). Published by Wolters Kluwer Health, Inc.
Conflict of interest statement
The authors have no conflicts to report.
Figures







References
-
- Devarbhavi H, Asrani SK, Arab JP, Nartey YA, Pose E, Kamath PS. Global burden of liver disease: 2023 update. J Hepatol. 2023;79:516–537. - PubMed
-
- Zuo T, Xie Q, Liu J, Yang J, Shi J, Kong D, et al. . Macrophage-derived cathepsin S remodels the extracellular matrix to promote liver fibrogenesis. Gastroenterology. 2023;165:746–761.e16. - PubMed
-
- Sinha S, Hassan N, Schwartz RE. Organelle stress and alterations in interorganelle crosstalk during liver fibrosis. Hepatology. 2024;79:482–501. - PubMed
-
- Fondevila MF, Fernandez U, Heras V, Parracho T, Gonzalez-Rellan MJ, Novoa E, et al. . Inhibition of carnitine palmitoyltransferase 1A in hepatic stellate cells protects against fibrosis. J Hepatol. 2022;77:15–28. - PubMed
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Medical
Miscellaneous

