Effect of rabbit ATG PK on outcomes after TCR-αβ/CD19-depleted pediatric haploidentical HCT for hematologic malignancy
- PMID: 39042892
- PMCID: PMC11629297
- DOI: 10.1182/bloodadvances.2024012670
Effect of rabbit ATG PK on outcomes after TCR-αβ/CD19-depleted pediatric haploidentical HCT for hematologic malignancy
Abstract
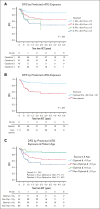
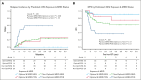
We hypothesized that the inferior disease-free survival (DFS) seen in older patients who underwent αβ-T-cell/CD19-depleted (AB-TCD) haploidentical hematopoietic cell transplantation (HCT) for hematologic malignancies is caused by excessive exposure to rabbit antithymocyte globulin (rATG; Thymoglobulin). Between 2015 and 2023, 163 patients with a median age of 13 years (range, 0.4-27.4) underwent AB-TCD haploidentical HCT for the treatment of acute lymphoblastic leukemia (n = 98), acute myeloid leukemia/myelodysplastic syndrome (n = 49), or other malignancies (n = 16) at 9 centers in 2 prospective trials. Exposures to rATG before and after HCT were predicted using a validated pharmacokinetic model. Receiver operating characteristic curves were used to identify the optimal target windows for rATG exposure in terms of outcomes. We identified 4 quadrants of rATG exposure, namely quadrant 1 (n = 52) with a high pre-HCT area under curve (AUC; ≥50 arbitrary units [AU] per day per milliliter) and a low post-HCT AUC (<12 AU per day per liter); quadrant 2 (n = 47) with a low pre- and post-HCT AUC; quadrant 3 (n = 13) with a low pre-HCT and a high post-HCT AUC; and quadrant 4 (n = 51) with a high pre- and post-HCT AUC. Quadrant 1 had a 3-year DFS of 86.5%, quadrant 2 had a DFS of 64.6%, quadrant 3 had a DFS of 32.9%, and for quadrant 4 it was 48.2%. An adjusted regression analysis demonstrated additional factors that were associated with an increased hazard for worse DFS, namely minimal residual disease (MRD) positivity and cytomegalovirus (CMV) R+/D- serostatus. Nonoptimal rATG exposure exhibited the strongest effect in unadjusted and adjusted (MRD status or CMV serostatus) analyses. High exposure to rATG after HCT was associated with inferior DFS following AB-TCD haploidentical HCT for pediatric patients with hematologic malignancies. Model-based dosing of rATG to achieve optimal exposure may improve DFS. These trials were registered at www.ClinicalTrials.gov as #NCT02646839 and #NCT04337515.
© 2024 by The American Society of Hematology. Licensed under Creative Commons Attribution-NonCommercial-NoDerivatives 4.0 International (CC BY-NC-ND 4.0), permitting only noncommercial, nonderivative use with attribution. All other rights reserved.
Conflict of interest statement
Conflict-of-interest disclosure: C.C.D. reports being a consultant for and serving on advisory boards of Jazz Pharmaceuticals, Alexion Inc, and AlloVir. J-A.T. reports receiving study support from Miltenyi. H.A.-A. reports serving on the advisory boards for Adaptive, Vertex, and Johnson & Johnson, and receiving study support from Adaptive. M.A.P. reports serving on advisory boards for Novartis, Equillium, bluebird bio, Medexus, Pfizer, GentiBio, and Vertex; engaging in educational activities for Novartis; and receiving study support from Miltenyi (not for this trial) and Adaptive. The remaining authors declare no competing financial interests.
Figures



References
-
- Locatelli F, Bauquet A, Palumbo G, Moretta F, Bertaina A. Negative depletion of α/β+ T cells and of CD19+ B lymphocytes: a novel frontier to optimize the effect of innate immunity in HLA-mismatched hematopoietic stem cell transplantation. Immunol Lett. 2013;155(1-2):21–23. - PubMed
-
- Bertaina A, Merli P, Rutella S, et al. HLA-haploidentical stem cell transplantation after removal of αβ+ T and B cells in children with nonmalignant disorders. Blood. 2014;124(5):822–826. - PubMed
-
- Locatelli F, Merli P, Pagliara D, et al. Outcome of children with acute leukemia given HLA-haploidentical HSCT after αβ T-cell and B-cell depletion. Blood. 2017;130(5):677–685. - PubMed
-
- Shah RM, Elfeky R, Nademi Z, et al. T-cell receptor αβ+ and CD19+ cell-depleted haploidentical and mismatched hematopoietic stem cell transplantation in primary immune deficiency. J Allergy Clin Immunol. 2018;141(4):1417–1426.e1. - PubMed

