T-cell dysfunction in CLL is mediated through expression of Siglec-10 ligands CD24 and CD52 on CLL cells
- PMID: 39042920
- PMCID: PMC11401197
- DOI: 10.1182/bloodadvances.2023011934
T-cell dysfunction in CLL is mediated through expression of Siglec-10 ligands CD24 and CD52 on CLL cells
Abstract
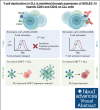
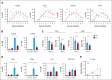
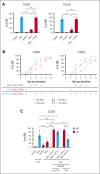
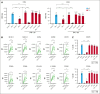
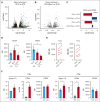
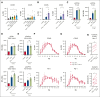
Autologous T-cell-based therapies, such as chimeric antigen receptor (CAR) T-cell therapy, exhibit low success rates in chronic lymphocytic leukemia (CLL) and correlate with a dysfunctional T-cell phenotype observed in patients. Despite various proposed mechanisms of T-cell dysfunction in CLL, the specific CLL-derived factors responsible remain unidentified. This study aimed to investigate the mechanisms through which CLL cells suppress CAR T-cell activation and function. We found that CLL-derived T cells get activated, albeit in a delayed fashion, and specifically that restimulation of CAR T cells in the presence of CLL cells causes impaired cytokine production and reduced proliferation. Notably, coculture of T cells with CD40-activated CLL cells did not lead to T-cell dysfunction, and this required direct cell contact between the CD40-stimulated CLL cells and T cells. Inhibition of kinases involved in the CD40 signaling cascade revealed that the Spare Respiratory Capacity (SRC) kinase inhibitor dasatinib prevented rescue of T-cell function independent of CD40-mediated increased levels of costimulatory and adhesion ligands on CLL cells. Transcriptome profiling of CD40-stimulated CLL cells with or without dasatinib identified widespread differential gene expression. Selecting for surface receptor genes revealed CD40-mediated downregulation of the Sialic acid-binding Ig-like lectin 10 (Siglec-10) ligands CD24 and CD52, which was prevented by dasatinib, suggesting a role for these ligands in functional T-cell suppression in CLL. Indeed, blocking CD24 and/or CD52 markedly reduced CAR T-cell dysfunction upon coculture with resting CLL cells. These results demonstrated that T cells derived from CLL patients can be reinvigorated by manipulating CLL-T-cell interactions. Targeting CD24- and CD52-mediated CLL-T-cell interaction could be a promising therapeutic strategy to enhance T-cell function in CLL.
© 2024 by The American Society of Hematology. Licensed under Creative Commons Attribution-NonCommercial-NoDerivatives 4.0 International (CC BY-NC-ND 4.0), permitting only noncommercial, nonderivative use with attribution. All other rights reserved.
Conflict of interest statement
Conflict-of-interest disclosure: The authors declare no competing financial interests.
Figures

References
-
- Killock D. Can FCR be curative in CLL? Nat Rev Clin Oncol. 2015;12(12):684. 684. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Molecular Biology Databases
Research Materials
Miscellaneous

