Adjuvant endocrine therapy choices in premenopausal patients with hormone receptor-positive early breast cancer: Insights from the prospective GIM23-POSTER study
- PMID: 39043079
- PMCID: PMC11325348
- DOI: 10.1016/j.breast.2024.103769
Adjuvant endocrine therapy choices in premenopausal patients with hormone receptor-positive early breast cancer: Insights from the prospective GIM23-POSTER study
Abstract
Background: Most premenopausal patients with early breast cancer (eBC) are diagnosed with hormone receptor-positive disease and therefore candidate for adjuvant endocrine therapy (ET).
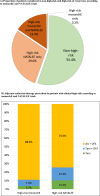
Patients and methods: The Gruppo Italiano Mammella (GIM) 23-POSTER (GIM23) is a multicenter, prospective, observational study conducted in 26 Italian institutions, aiming to evaluate ET choices for premenopausal patients affected by hormone receptor-positive eBC in a real-world setting. Here we report also the results in terms of type of ET prescribed according to the definition of high-risk patients by monarchE and NATALEE trials.
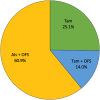
Results: Between October 2019 and June 2022, 600 premenopausal patients were included, with a median age of 46 years. Almost half (271, 45.2 %) of the patients had stage I disease, while 254 (42.3 %) and 60 (10.0 %) patients had stage II and III, respectively. Overall, 149 (25.1 %) patients received tamoxifen alone, 83 (14.0 %) tamoxifen with ovarian function suppression (OFS), while 361 (60.9 %) received aromatase inhibitor (AI) with OFS. Patients treated with AI and OFS had higher number of metastatic axillary nodes, higher grade and more often received chemotherapy (all p < 0.001). According to the inclusion criteria of the monarchE and NATALEE trials, 81 patients (15.6 %) were considered high-risk for the monarchE and received AI with OFS in 88.9 % of the cases, while 231 patients (44.4 %) were considered high-risk for the NATALEE trial and received AI with OFS in 74.5 % of cases.
Conclusions: AI with OFS is the most prescribed adjuvant ET among premenopausal patients, especially in the presence of high-risk features.
Keywords: Adjuvant endocrine therapy; Aromatase inhibitors; Breast cancer; CDK4/6-Inhibitors; Ovarian function suppression; Premenopausal patients; Tamoxifen.
Copyright © 2024 The Authors. Published by Elsevier Ltd.. All rights reserved.
Conflict of interest statement
Declaration of competing interest The authors declare the following financial interests/personal relationships which may be considered as potential competing interests: Eva Blondeaux: speaker fee from Eli Lilly and research support from Gilead Science (to the institution). Valentina Guarneri: personal fees from EliLilly, Exact Sciences, Novartis, Pfizer, Gilead, MSD, Amgen, Sanofi, Merck Serono, and Eisai outside the submitted work. Eleonora Mioranza: personal fee from Novartis e Lilly. Fabio Puglisi: advisory role for and receiving speaker honoraria from Amgen, AstraZeneca, Daichii Sankyo, Celgene, Eisai, Eli Lilly, Exact Sciences, Gilead, Ipsen, Menarini, MSD, Novartis, Pierre Fabre, Pfizer, Roche, Seagen, Takeda, and Viatris. Travel grants from AstraZeneca, Daichii Sankyo, Novartis, Roche. Research grants (to his institution) from Astrazeneca, Eisai, Roche. Antonella Ferro: support for attending meeting from Gilead, MSD and Pfizer; honoraria from Pfizer, Novartis, Daiichi Sankyo, Ely Lilly, Seagen, Gilead, Astra Zeneca, MSD. Vincenzo Adamo: consultancy/advisory role/speaker bureau from Amgen, Astra Zeneca, BMS, Gilead, Lilly, MSD, Novartis, Pfizer, Roche, Sandoz, Sanofi, Sevier, Takeda. Laura Biganzoli: Personal financial interests (Honoraria, consultancy or advisory role): Amgen, AstraZeneca, Boehringer-Ingelheim, Daiichi-Sankyo, Eisai, Exact Sciences, Gilead, Lilly, Menarini, Novartis, Pfizer, Pierre Fabre, Roche, Sanofi, SeaGen. Institutional financial interests: Celgene, Genomic Health, Novartis. Travel grant: AstraZeneca, Daiichi-Sankyo. Evandro de Azambuja: honoraria from or participation in advisory boards for Roche, Genentech, Novartis, SeaGen, Zodiac, Libbs, Pierre Fabre, Eli Lilly, and AstraZeneca. Francesca Poggio: fees and other support from AstraZeneca and personal fees from Eli Lilly, Novartis, Daichii Sankyo, and Gilead outside the submitted work. Matteo Lambertini: advisory role for Roche, Lilly, Novartis, AstraZeneca, Pfizer, Seagen, Gilead, MSD, and Exact Sciences; receiving speaker honoraria from Roche, Lilly, Novartis, Pfizer, Sandoz, Libbs, Daiichi Sankyo, Takeda, Knight, Ipsen, and AstraZeneca; receiving travel grants from Gilead and Daiichi Sankyo; receiving research funding (to his institution) from Gilead. Lucia Del Mastro: grants from Eli Lilly, Novartis, Roche, Daiichi Sankyo, Seagen, AstraZeneca, Gilead, and Pierre Fabre; receiving consulting fees from Eli Lilly, Gilead, and Daiichi Sankyo; receiving speaker honoraria from Roche, Novartis, Pfizer, Eli Lilly, AstraZeneca, MSD, Seagen, Gilead, Pierre Fabre, Eisai, Exact Sciences, Ipsen, GSK, and Agendia-Stemline; receiving travel grants from Roche, Pfizer, Eisai, Daiichi Sankyo, and AstraZeneca; and having an advisory role for Novartis, Roche, Eli Lilly, Pfizer, Daiichi Sakyo, Exact Sciences, Gilead, Pierre Fabre, Eisai, AstraZeneca, Agendia, GSK, and Seagen. All the other authors declare no conflict of interest.
Figures

References
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Medical

