First Detection of Antibodies Specific to Crimean-Congo Hemorrhagic Fever Virus in Rural Populations of Gabon
- PMID: 39043170
- PMCID: PMC11448543
- DOI: 10.4269/ajtmh.24-0054
First Detection of Antibodies Specific to Crimean-Congo Hemorrhagic Fever Virus in Rural Populations of Gabon
Abstract
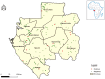
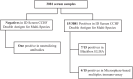
Crimean-Congo hemorrhagic fever (CCHF) is a tick-borne viral disease with a mortality rate reaching up to 40% in humans. Currently, CCHF affects three continents: Asia, Europe, and Africa. An increase in confirmed cases in Africa has been observed since 2000. In Central Africa, several countries have reported the circulation of CCHV virus (CCHFV). However, in Gabon, there is a lack of recent data on the circulation of the virus in the Gabonese population. To provide an overview of the epidemiological situation in Gabon, we tested 3,081 human serum samples collected between 2005 and 2008 in villages throughout the country for anti-CCHFV antibodies. Using a double-antigen ELISA kit, our study found 15/3,081 samples positive for CCHFV. These positive samples were also tested using the Blackbox CCHFV IgG kit and the Luminex technique. These analyses confirmed seven and four positives for the Blackbox CCHFV IgG kit and the Luminex technique, respectively. This study suggests low circulation of CCHFV in the rural human population of Gabon. Competent authorities must survey CCHFV to identify and prevent clinical cases in the human population.
Conflict of interest statement
Disclosures: The study was conducted in accordance with the Declaration of Helsinki and approved by the Gabonese Ministry of Health (research authorization 00093/MSP/SG/SGAQM). Informed consent was obtained from all subjects involved in the study. The funders had no role in the design of the study, in the collection, analyses, or interpretation of the data, in the writing of the manuscript, or in the decision to publish the results.
Authors’ contributions: Conceptualization, P. Becquart, S. Lerolle, and V. Legros; methodology, P. Becquart, E. M. Leroy, and G. D. Maganga; validation, N. N’dilimabaka, V. Legros, F.-L. Cosset, and P. Becquart; formal analysis, L. Bohou Kombila, S. Lerolle, V. Legros, N.-M. Longo-Pendy, and D. Koumba Mavoungou; investigation, L. Bohou Kombila, S. Lerolle, and D. Koumba Mavoungou; data curation, L. Bohou Kombila, S. Lerolle, V, Legros, and F.-L. Cosset; writing—original draft preparation, L. Bohou Kombila and S. Lerolle; writing—review and editing, P. Becquart, I. M. Mombo, J. Vanhomwegen, S. Lerolle, V. Legros, C. Deschermeier, F.-L. Cosset, and N. N’dilimabaka; supervision, P. Becquart, N. N’dilimabaka, V. Legros, and F.-L. Cosset; project administration, P. Becquart, E. M. Leroy, V. Legros, and N. N’dilimabaka; funding acquisition, V. Legros, F.-L. Cosset, and E. M. Leroy. All authors have read and approved the published version of the manuscript.
Figures

References
-
- Spengler JR, Estrada-Peña A, Garrison AR, Schmaljohn C, Spiropoulou CF, Bergeron É, Bente DA, 2016. A chronological review of experimental infection studies of the role of wild animals and livestock in the maintenance and transmission of Crimean-Congo hemorrhagic fever virus. Antiviral Res 135: 31–47. - PMC - PubMed
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Medical

