A highly potent bi-thiazole inhibitor of LOX rewires collagen architecture and enhances chemoresponse in triple-negative breast cancer
- PMID: 39043186
- PMCID: PMC11585458
- DOI: 10.1016/j.chembiol.2024.06.012
A highly potent bi-thiazole inhibitor of LOX rewires collagen architecture and enhances chemoresponse in triple-negative breast cancer
Abstract
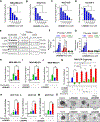
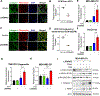
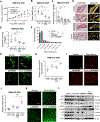
Lysyl oxidase (LOX) is upregulated in highly stiff aggressive tumors, correlating with metastasis, resistance, and worse survival; however, there are currently no potent, safe, and orally bioavailable small molecule LOX inhibitors to treat these aggressive desmoplastic solid tumors in clinics. Here we discovered bi-thiazole derivatives as potent LOX inhibitors by robust screening of drug-like molecules combined with cell/recombinant protein-based assays. Structure-activity relationship analysis identified a potent lead compound (LXG6403) with ∼3.5-fold specificity for LOX compared to LOXL2 while not inhibiting LOXL1 with a competitive, time- and concentration-dependent irreversible mode of inhibition. LXG6403 shows favorable pharmacokinetic properties, globally changes ECM/collagen architecture, and reduces tumor stiffness. This leads to better drug penetration, inhibits FAK signaling, and induces ROS/DNA damage, G1 arrest, and apoptosis in chemoresistant triple-negative breast cancer (TNBC) cell lines, PDX organoids, and in vivo. Overall, our potent and tolerable bi-thiazole LOX inhibitor enhances chemoresponse in TNBC, the deadliest breast cancer subtype.
Keywords: ECM; FAK; LOX inhibitor; ROS; TNBC chemoresistance; collagen architecture; lysyl oxidase.
Copyright © 2024 The Author(s). Published by Elsevier Ltd.. All rights reserved.
Conflict of interest statement
Declaration of interests O. Sahin is the co-founder and manager of OncoCube Therapeutics LLC, founder and president of LoxiGen, and member of scientific advisory board of A2A Pharmaceuticals Inc. M.C., O. Saatci, A.-H.R., C.B., C.M., and O.S. are inventors on patent US 11,712,437.
Figures



References
-
- Miller BW, Morton JP, Pinese M, Saturno G, Jamieson NB, McGhee E, Timpson P, Leach J, McGarry L Shanks E (2015). Targeting the LOX/hypoxia axis reverses many of the features that make pancreatic cancer deadly: inhibition of LOX abrogates metastasis and enhances drug efficacy. EMBO molecular medicine. 7: 1063–1076 - PMC - PubMed
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Miscellaneous

