Sleep-wake behavior and responses to sleep deprivation and immune challenge of protein kinase RNA-activated knockout mice
- PMID: 39043346
- PMCID: PMC11563030
- DOI: 10.1016/j.bbi.2024.07.027
Sleep-wake behavior and responses to sleep deprivation and immune challenge of protein kinase RNA-activated knockout mice
Abstract
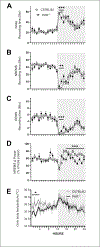
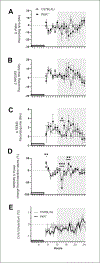
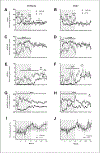
Protein Kinase RNA-activated (PKR) is an enzyme that plays a role in many systemic processes, including modulation of inflammation, and is implicated in neurodegenerative diseases, such as Alzheimer's disease (AD). PKR phosphorylation results in the production of several cytokines involved in the regulation / modulation of sleep, including interleukin-1β, tumor necrosis factor-α and interferon-γ. We hypothesized targeting PKR would alter spontaneous sleep of mice, attenuate responses to sleep deprivation, and inhibit responses to immune challenge. To test these hypotheses, we determined the sleep-wake phenotype of mice lacking PKR (knockout; PKR-/-) during undisturbed baseline conditions; in responses to six hours of sleep deprivation; and after immune challenge with lipopolysaccharide (LPS). Adult male mice (C57BL/6J, n = 7; PKR-/-, n = 7) were surgically instrumented with EEG recording electrodes and an intraperitoneal microchip to record core body temperature. During undisturbed baseline conditions, PKR -/- mice spent more time in non-rapid eye movement sleep (NREMS) and rapid-eye movement sleep (REMS), and less time awake at the beginning of the dark period of the light:dark cycle. Delta power during NREMS, a measure of sleep depth, was less in PKR-/- mice during the dark period, and core body temperatures were lower during the light period. Both mouse strains responded to sleep deprivation with increased NREMS and REMS, although these changes did not differ substantively between strains. The initial increase in delta power during NREMS after sleep deprivation was greater in PKR-/- mice, suggesting a faster buildup of sleep pressure with prolonged waking. Immune challenge with LPS increased NREMS and inhibited REMS to the same extent in both mouse strains, whereas the initial LPS-induced suppression of delta power during NREMS was greater in PKR-/- mice. Because sleep regulatory and immune responsive systems in brain are redundant and overlapping, other mediators and signaling pathways in addition to PKR are involved in the responses to acute sleep deprivation and LPS immune challenge.
Keywords: Alzheimer’s disease; Cytokines; Inflammation; Lipopolysaccharide; Neurodegeneration; PKR; Sleep; Sleep deprivation.
Copyright © 2024 The Authors. Published by Elsevier Inc. All rights reserved.
Conflict of interest statement
Declaration of Competing Interest The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
Figures




Similar articles
-
Sleep-wake behavior and responses to sleep deprivation of mice lacking both interleukin-1 beta receptor 1 and tumor necrosis factor-alpha receptor 1.Brain Behav Immun. 2008 Aug;22(6):982-93. doi: 10.1016/j.bbi.2008.02.001. Epub 2008 Mar 7. Brain Behav Immun. 2008. PMID: 18329246 Free PMC article.
-
Cyclooxygenase-2 (COX-2)-dependent mechanisms mediate sleep responses to microbial and thermal stimuli.Brain Behav Immun. 2024 Nov;122:325-338. doi: 10.1016/j.bbi.2024.08.014. Epub 2024 Aug 10. Brain Behav Immun. 2024. PMID: 39134184
-
Ageing-related modification of sleep and breathing in orexin-knockout narcoleptic mice.J Sleep Res. 2025 Apr;34(2):e14287. doi: 10.1111/jsr.14287. Epub 2024 Jul 20. J Sleep Res. 2025. PMID: 39032099 Free PMC article.
-
The Black Book of Psychotropic Dosing and Monitoring.Psychopharmacol Bull. 2024 Jul 8;54(3):8-59. Psychopharmacol Bull. 2024. PMID: 38993656 Free PMC article. Review.
-
Pharmacotherapies for sleep disturbances in dementia.Cochrane Database Syst Rev. 2016 Nov 16;11(11):CD009178. doi: 10.1002/14651858.CD009178.pub3. Cochrane Database Syst Rev. 2016. Update in: Cochrane Database Syst Rev. 2020 Nov 15;11:CD009178. doi: 10.1002/14651858.CD009178.pub4. PMID: 27851868 Free PMC article. Updated.
Cited by
-
Interferon Regulatory Factors as a Potential Therapeutic Target for Neuroinflammation: A Focus on Alzheimer's Disease.Int J Mol Sci. 2025 Mar 23;26(7):2906. doi: 10.3390/ijms26072906. Int J Mol Sci. 2025. PMID: 40243463 Free PMC article. Review.
-
Dynamic changes in peripheral inflammation as a risk factor for perioperative sleep disturbances in elderly patients undergoing laparoscopic hepatobiliary surgery.Front Neurol. 2025 Apr 16;16:1537780. doi: 10.3389/fneur.2025.1537780. eCollection 2025. Front Neurol. 2025. PMID: 40308223 Free PMC article.
-
Dynamic changes in sleep architecture in a mouse model of acute kidney injury transitioning to chronic kidney disease.Front Neurosci. 2025 Jul 3;19:1581494. doi: 10.3389/fnins.2025.1581494. eCollection 2025. Front Neurosci. 2025. PMID: 40678758 Free PMC article.
References
-
- Abraham N, Stojdl DF, Duncan PI, Méthot N, Ishii T, Dubé M, Vanderhyden BC, Atkins HL, Gray DA, McBurney MW, Koromilas AE, Brown EG, Sonenberg N, Bell JC, 1999. Characterization of transgenic mice with targeted disruption of the catalytic domain of the double-stranded RNA-dependent protein kinase, PKR. J. Biol. Chem. 274, 5953–5962. 10.1074/jbc.274.9.5953. - DOI - PubMed
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources

