Learning implementation of a guideline based decision support system to improve hypertension treatment in primary care in China: pragmatic cluster randomised controlled trial
- PMID: 39043397
- PMCID: PMC11265211
- DOI: 10.1136/bmj-2023-079143
Learning implementation of a guideline based decision support system to improve hypertension treatment in primary care in China: pragmatic cluster randomised controlled trial
Abstract
Objective: To evaluate the effectiveness of a clinical decision support system (CDSS) in improving the use of guideline accordant antihypertensive treatment in primary care settings in China.
Design: Pragmatic, open label, cluster randomised trial.
Setting: 94 primary care practices in four urban regions of China between August 2019 and July 2022: Luoyang (central China), Jining (east China), and Shenzhen (south China, including two regions).
Participants: 94 practices were randomised (46 to CDSS, 48 to usual care). 12 137 participants with hypertension who used up to two classes of antihypertensives and had a systolic blood pressure <180 mm Hg and diastolic blood pressure <110 mm Hg were included.
Interventions: Primary care practices were randomised to use an electronic health record based CDSS, which recommended a specific guideline accordant regimen for initiation, titration, or switching of antihypertensive (the intervention), or to use the same electronic health record without CDSS and provide treatment as usual (control).
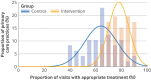
Main outcome measures: The primary outcome was the proportion of hypertension related visits during which an appropriate (guideline accordant) treatment was provided. Secondary outcomes were the average reduction in systolic blood pressure and proportion of participants with controlled blood pressure (<140/90 mm Hg) at the last scheduled follow-up. Safety outcomes were patient reported antihypertensive treatment related events, including syncope, injurious fall, symptomatic hypotension or systolic blood pressure <90 mm Hg, and bradycardia.
Results: 5755 participants with 23 113 visits in the intervention group and 6382 participants with 27 868 visits in the control group were included. Mean age was 61 (standard deviation 13) years and 42.5% were women. During a median 11.6 months of follow-up, the proportion of visits at which appropriate treatment was given was higher in the intervention group than in the control group (77.8% (17 975/23 113) v 62.2% (17 328/27 868); absolute difference 15.2 percentage points (95% confidence interval (CI) 10.7 to 19.8); P<0.001; odds ratio 2.17 (95% CI 1.75 to 2.69); P<0.001). Compared with participants in the control group, those in the intervention group had a 1.6 mm Hg (95% CI -2.7 to -0.5) greater reduction in systolic blood pressure (-1.5 mm Hg v 0.3 mm Hg; P=0.006) and a 4.4 percentage point (95% CI -0.7 to 9.5) improvement in blood pressure control rate (69.0% (3415/4952) v 64.6% (3778/5845); P=0.07). Patient reported antihypertensive treatment related adverse effects were rare in both groups.
Conclusions: Use of a CDSS in primary care in China improved the provision of guideline accordant antihypertensive treatment and led to a modest reduction in blood pressure. The CDSS offers a promising approach to delivering better care for hypertension, both safely and efficiently.
Trial registration: ClinicalTrials.gov NCT03636334.
© Author(s) (or their employer(s)) 2019. Re-use permitted under CC BY-NC. No commercial re-use. See rights and permissions. Published by BMJ.
Conflict of interest statement
Competing interests: All authors have completed the ICMJE uniform disclosure form at https://www.icmje.org/disclosure-of-interest/ and declare: support from the Chinese Academy of Medical Sciences and the Sanming Project of Medicine in Shenzhen; in the past three years, HMK received options for Element Science and Identifeye and payments from F-Prime for advisory roles, and he is a cofounder of, and holds equity in, Hugo Health, Refactor Health, and Ensight-AI, and he is associated with research contracts through Yale University from Janssen, Kenvue, and Pfizer; no other relationships or activities that could appear to have influenced the submitted work.
Figures


Comment in
-
Improving hypertension management in primary care.BMJ. 2024 Jul 23;386:q1466. doi: 10.1136/bmj.q1466. BMJ. 2024. PMID: 39043414 No abstract available.
References
-
- Zhou B, Carrillo-Larco RM, Danaei G, et al. NCD Risk Factor Collaboration (NCD-RisC) . Worldwide trends in hypertension prevalence and progress in treatment and control from 1990 to 2019: a pooled analysis of 1201 population-representative studies with 104 million participants. Lancet 2021;398:957-80. 10.1016/S0140-6736(21)01330-1 - DOI - PMC - PubMed
Publication types
MeSH terms
Substances
Associated data
LinkOut - more resources
Full Text Sources
Medical
