Efficacy and safety of faricimab for neovascular age-related macular degeneration: a systematic review and network meta-analysis
- PMID: 39043575
- PMCID: PMC11268043
- DOI: 10.1136/bmjophth-2024-001702
Efficacy and safety of faricimab for neovascular age-related macular degeneration: a systematic review and network meta-analysis
Abstract
Objective: To evaluate the efficacy and safety of faricimab compared with other anti-vascular endothelial growth factor (anti-VEGF) agents in treating neovascular age-related macular degeneration (nAMD) patients.
Methods and analysis: A systematic review (SR) was conducted up to January 2023. Network meta-analyses (NMA) were performed, including sensitivity and subgroup analyses for naïve population. Outcomes included changes in visual acuity (Early Treatment of Diabetic Retinopathy Study [ETDRS] letters), anatomical changes, frequency of injections and adverse events. The Cochrane Collaboration guidelines and the Confidence in Network Meta-Analysis framework were used for the SR and the certainty of evidence, respectively.
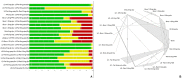
Results: From 4128 identified records through electronic databases and complementary searches, 63 randomised controlled trials (RCTs) met the eligibility criteria, with 42 included in the NMA. Faricimab showed a significant reduction in the number of annual injections compared with most fixed and flexible anti-VEGF treatment regimens, while showing no statistically significant differences in visual acuity through ETDRS letter gain, demonstrating a comparable efficacy. Retinal thickness results showed comparable efficacy to other anti-VEGF agents, and inferior only to brolucizumab. Results also showed that more patients treated with faricimab were free from post-treatment retinal fluid compared with aflibercept every 8 weeks, and both ranibizumab and bevacizumab, in the fixed and pro re nata (PRN) assessed schedules. Faricimab showed a comparable safety profile regarding the risk of ocular adverse events and serious ocular adverse events (SOAE), except for the comparison with brolucizumab quarterly, in which faricimab showed a significant reduction for SOAE risk.
Conclusion: Faricimab showed a comparable clinical benefit in efficacy and safety outcomes, with a reduction in annual injections compared with fixed and flexible anti-VEGF drug regimens, representing a valuable treatment option for nAMD patients.
Prospero registration number: CRD42023394226.
Keywords: Macula; Neovascularisation; Retina; Treatment Medical.
© Author(s) (or their employer(s)) 2024. Re-use permitted under CC BY-NC. No commercial re-use. See rights and permissions. Published by BMJ.
Conflict of interest statement
Competing interests: RF, CS and OH have declared financial support from pharmaceutical companies, including Bayer, Novartis, Astellas, Abbvie and Roche, for conferences and academic meetings, as well as for consulting fees and Advisory Boards. DS-S, P-PL and KJ are employees of Roche, Colombia. None of the authors received any compensation for the authorship of this manuscript. IQVIA served as consultant company for Roche for the development of the study. The authors have no other relevant affiliations or financial involvement with any organisation or entity with a financial interest in or financial conflict with the subject matter or materials discussed in the manuscript apart from those disclosed.
Figures


References
-
- World Health Organization (WHO) Vision 2020 the right to sight. Global iniciative for the elimination of avoidable blindness. action plan 2006-2011. 2007:1–89.
-
- Keenan TD, Cukras CA, Chew EY. Age-related Macular Degeneration. vol 1256. Springer, Cham; 2021. Age-related macular degeneration: epidemiology and clinical aspects. - PubMed
-
- Lanzetta P, Loewenstein A, Vision Academy Steering C. Fundamental principles of an anti-VEGF treatment regimen: optimal application of intravitreal anti-vascular endothelial growth factor therapy of macular diseases. Graefes Arch Clin Exp Ophthalmol. 2017;255:1259–73. doi: 10.1007/s00417-017-3647-4. - DOI - PMC - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Research Materials
Miscellaneous
