Directing B7-H3 chimeric antigen receptor T cell homing through IL-8 induces potent antitumor activity against pediatric sarcoma
- PMID: 39043604
- PMCID: PMC11268054
- DOI: 10.1136/jitc-2024-009221
Directing B7-H3 chimeric antigen receptor T cell homing through IL-8 induces potent antitumor activity against pediatric sarcoma
Abstract
Background: Advances in pediatric oncology have occurred for some cancers; however, new therapies for sarcoma have been inadequate. Cellular immunotherapy using chimeric antigen receptor (CAR) T cells has shown dramatic benefits in leukemia, lymphoma, and multiple myeloma but has been far less successful in pediatric solid tumors such as rhabdomyosarcoma (RMS) and osteosarcoma (OS). Balancing issues of "on-target, off-tumor toxicity", investigators have identified B7-H3 as a broadly expressed tumor antigen with otherwise restricted expression on normal tissues. We hypothesized that rapid homing via a chemokine receptor and CAR engagement through B7-H3 would enhance CAR T cell efficacy in solid tumors.
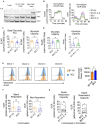
Methods: We generated B7-H3 CAR T cells that also express the Interleukin-8 (IL-8) receptor, CXCR2. Cytokine production, flow cytometry, Seahorse assays and RNA sequencing were used to compare the B7-H3 CXCR2 (BC2) CAR T cells with B7-H3 CAR T cells. We developed an IL-8 overexpressing human RMS mouse model to test homing and cytotoxicity in vivo.
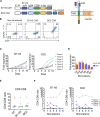
Results: We demonstrate that IL-8 is expressed by RMS and OS and expression significantly increases after radiation. Overexpression of an IL-8 receptor, CXCR2, on B7-H3 CAR T cells enhances homing into IL-8 expressing tumors, augments T cell metabolism and leads to significant tumor regression.
Conclusion: These findings warrant further investigation into the use of BC2 CAR T cells as a treatment for patients with RMS, OS and other B7-H3-expressing, IL-8 producing solid tumors.
Keywords: Adoptive cell therapy - ACT; Chimeric antigen receptor - CAR; Immunotherapy; Solid tumor.
© Author(s) (or their employer(s)) 2024. Re-use permitted under CC BY-NC. No commercial re-use. See rights and permissions. Published by BMJ.
Conflict of interest statement
Competing interests: US Patent (University of Colorado): W02022221592: Compositions and methods for producing and using cell-based immunotherapies to target tumors (inventors: MRV and JAL).
Figures


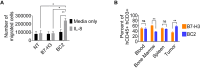

References
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Research Materials
