Obesity increases genomic instability at DNA repeat-mediated endogenous mutation hotspots
- PMID: 39043652
- PMCID: PMC11266421
- DOI: 10.1038/s41467-024-50006-8
Obesity increases genomic instability at DNA repeat-mediated endogenous mutation hotspots
Abstract
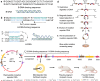
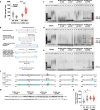
Obesity is associated with increased cancer risk, yet the underlying mechanisms remain elusive. Obesity-associated cancers involve disruptions in metabolic and cellular pathways, which can lead to genomic instability. Repetitive DNA sequences capable of adopting alternative DNA structures (e.g., H-DNA) stimulate mutations and are enriched at mutation hotspots in human cancer genomes. However, it is not known if obesity impacts DNA repeat-mediated endogenous mutation hotspots. We address this gap by measuring mutation frequencies in obese and normal-weight transgenic reporter mice carrying either a control human B-DNA- or an H-DNA-forming sequence (from a translocation hotspot in c-MYC in Burkitt lymphoma). Here, we discover that H-DNA-induced DNA damage and mutations are elevated in a tissue-specific manner, and DNA repair efficiency is reduced in obese mice compared to those on the control diet. These findings elucidate the impact of obesity on cancer-associated endogenous mutation hotspots, providing mechanistic insight into the link between obesity and cancer.
© 2024. The Author(s).
Conflict of interest statement
The authors declare no competing interests.
Figures






References
-
- Mannar M., Micha R. 2020 Global Nutrition Report: Action On Equity To End Malnutrition (Development Initiatives, 2020).
-
- The Lancet Diabetes and Endocrinology. The obesity-cancer link: of increasing concern. Lancet Diabetes Endocrinol.8, 175 (2020). - PubMed
MeSH terms
Substances
Grants and funding
- R01 CA093729/CA/NCI NIH HHS/United States
- RP170002/Cancer Prevention and Research Institute of Texas (Cancer Prevention Research Institute of Texas)
- CA093729/U.S. Department of Health & Human Services | NIH | National Cancer Institute (NCI)
- CA225029/U.S. Department of Health & Human Services | NIH | National Cancer Institute (NCI)
- R01 CA225029/CA/NCI NIH HHS/United States
LinkOut - more resources
Full Text Sources
Medical
Miscellaneous

