Vitamin B5 metabolism is essential for vacuolar and mitochondrial functions and drug detoxification in fungi
- PMID: 39043829
- PMCID: PMC11266677
- DOI: 10.1038/s42003-024-06595-7
Vitamin B5 metabolism is essential for vacuolar and mitochondrial functions and drug detoxification in fungi
Abstract
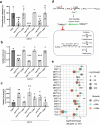
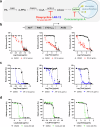
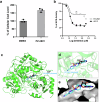
Fungal infections, a leading cause of mortality among eukaryotic pathogens, pose a growing global health threat due to the rise of drug-resistant strains. New therapeutic strategies are urgently needed to combat this challenge. The PCA pathway for biosynthesis of Co-enzyme A (CoA) and Acetyl-CoA (AcCoA) from vitamin B5 (pantothenic acid) has been validated as an excellent target for the development of new antimicrobials against fungi and protozoa. The pathway regulates key cellular processes including metabolism of fatty acids, amino acids, sterols, and heme. In this study, we provide genetic evidence that disruption of the PCA pathway in Saccharomyces cerevisiae results in a significant alteration in the susceptibility of fungi to a wide range of xenobiotics, including clinically approved antifungal drugs through alteration of vacuolar morphology and drug detoxification. The drug potentiation mediated by genetic regulation of genes in the PCA pathway could be recapitulated using the pantazine analog PZ-2891 as well as the celecoxib derivative, AR-12 through inhibition of fungal AcCoA synthase activity. Collectively, the data validate the PCA pathway as a suitable target for enhancing the efficacy and safety of current antifungal therapies.
© 2024. The Author(s).
Conflict of interest statement
The authors declare the following competing interests. C.B.M. is the founder of Curatix, which focuses on the development of anti-infectives. J.C.Y. conducted this work while an Associate Research Scientist at Yale. He is currently a Scientific Director Curatix. All other authors declare that they have no conflict of interest with the content of this article. A patent application on the use of the PAMS strategy to potentiate antifungal drugs has been submitted by CBM.
Figures





References
Publication types
MeSH terms
Substances
Grants and funding
- R35 GM131701/GM/NIGMS NIH HHS/United States
- R44 AI136118/AI/NIAID NIH HHS/United States
- AI123321, AI138139, AI152220, and AI136118/U.S. Department of Health & Human Services | NIH | National Institute of Allergy and Infectious Diseases (NIAID)
- R01 AI123321/AI/NIAID NIH HHS/United States
- R01 AI138139/AI/NIAID NIH HHS/United States
LinkOut - more resources
Full Text Sources
Research Materials

