Improved outcome of COVID-19 over time in patients treated with CAR T-cell therapy: Update of the European COVID-19 multicenter study on behalf of the European Society for Blood and Marrow Transplantation (EBMT) Infectious Diseases Working Party (IDWP) and the European Hematology Association (EHA) Lymphoma Group
- PMID: 39043963
- PMCID: PMC11347385
- DOI: 10.1038/s41375-024-02336-1
Improved outcome of COVID-19 over time in patients treated with CAR T-cell therapy: Update of the European COVID-19 multicenter study on behalf of the European Society for Blood and Marrow Transplantation (EBMT) Infectious Diseases Working Party (IDWP) and the European Hematology Association (EHA) Lymphoma Group
Abstract
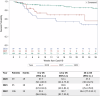
COVID-19 has been associated with high mortality in patients treated with Chimeric Antigen Receptor (CAR) T-cell therapy for hematologic malignancies. Here, we investigated whether the outcome has improved over time with the primary objective of assessing COVID-19-attributable mortality in the Omicron period of 2022 compared to previous years. Data for this multicenter study were collected using the MED-A and COVID-19 report forms developed by the EBMT. One-hundred-eighty patients were included in the analysis, 39 diagnosed in 2020, 35 in 2021 and 106 in 2022. The median age was 58.9 years (min-max: 5.2-78.4). There was a successive decrease in COVID-19-related mortality over time (2020: 43.6%, 2021: 22.9%, 2022: 7.5%) and in multivariate analysis year of infection was the strongest predictor of survival (p = 0.0001). Comparing 2022 with 2020-2021, significantly fewer patients had lower respiratory symptoms (21.7% vs 37.8%, p = 0.01), needed oxygen support (25.5% vs 43.2%, p = 0.01), or were admitted to ICU (5.7% vs 33.8%, p = 0.0001). Although COVID-19-related mortality has decreased over time, CAR T-cell recipients remain at higher risk for complications than the general population. Consequently, vigilant monitoring for COVID-19 in patients undergoing B-cell-targeting CAR T-cell treatment is continuously recommended ensuring optimal prevention of infection and advanced state-of-the art treatment when needed.
© 2024. The Author(s).
Conflict of interest statement
There is no financial support for this work that could have influenced the outcomes described in the manuscript. However, several authors report a potential conflict of interest, which is described below. PL has received speaker fees for Kite/Gilead and Pfizer, PB received advisory board and consultancy fees from Allogene, Amgen, BMS/Celgene, Kite/Gilead, Incyte, Miltenyi Biomedicine, Novartis, Nektar, Pfizer and Pierre Fabre, JM has received research support from Pfizer and Gilead and has served as a consultant or speaker for Gilead, Pfizer, MSD, Cidara, Mundipharma, F2G, Amplyx, Basilea, Shionoghi, Synexis and Takeda, AB has received fees for conference attendance and advisory boards from Kite/Gilead and consultancy fees from NovartisSLP participated in advisory boards for Novartis, Kite-Gilead and Janssen, MJK reports honoraria from BMS/Celgene, Kite/Gilead, Novartis, and Roche; consulting or advisory roles for BMS/Celgene, Kite Gilead, Miltenyi Biotec, Novartis, Takeda Pharmaceuticals, and Adicet Bio; and research funding from Kite Gilead, all to her institution. RC has received speaker fees for MSD, GSK, Novartis, Pfizer, Gilead and received advisory board and consultancy fees from MSD, Astellas, Roche; SM has received speaker’s fees via his institution from Celgene/BMS, Novartis, Janssen and Pfizer, received travel support and fees via his institution for participation in an expert panel from Kite/Gilead, served on DSMB for Miltenyi and Mendes (via his institution), is the founder of SWECARNET (via his institution) and his spouse is the founder of ScientifyResearch.
Figures
References
-
- Spanjaart AM, Ljungman P, de La Camara R, Tridello G, Ortiz-Maldonado V, Urbano-Ispizua A, et al. Poor outcome of patients with COVID-19 after CAR T-cell therapy for B-cell malignancies: results of a multicenter study on behalf of the European Society for Blood and Marrow Transplantation (EBMT) Infectious Diseases Working Party and the European Hematology Association (EHA) Lymphoma Group. Leukemia. 2021;35:3585–8. 10.1038/s41375-021-01466-0 - DOI - PMC - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Medical