Iron Reduces the Trafficking of Fatty Acids from Human Immortalised Brain Microvascular Endothelial Cells Through Modulation of Fatty Acid Transport Protein 1 (FATP1/SLC27A1)
- PMID: 39044044
- PMCID: PMC11362236
- DOI: 10.1007/s11095-024-03743-w
Iron Reduces the Trafficking of Fatty Acids from Human Immortalised Brain Microvascular Endothelial Cells Through Modulation of Fatty Acid Transport Protein 1 (FATP1/SLC27A1)
Abstract
Purpose: Alzheimer's disease (AD) is associated with brain accumulation of amyloid-beta (Aβ) and neurofibrillary tangle formation, in addition to reduced brain docosahexaenoic acid (DHA) and increased brain iron levels. DHA requires access across the blood-brain barrier (BBB) to enter the brain, and iron has been shown to affect the expression and function of a number of BBB transporters. Therefore, this study aimed to assess the effect of iron on the expression and function of fatty acid binding protein 5 (FABP5) and fatty acid transport protein 1 (FATP1), both which mediate brain endothelial cell trafficking of DHA.
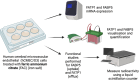
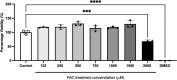
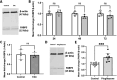
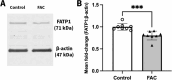
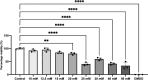
Methods: The mRNA and protein levels of FABP5 and FATP1 in human cerebral microvascular endothelial (hCMEC/D3) cells was assessed by RT-qPCR and Western blot, respectively following ferric ammonium citrate (FAC) treatment (up to 750 µM, 72 h). The function of FABP5 and FATP1 was assessed via uptake and efflux of radiolabelled 3H-oleic acid and 14C-DHA.
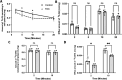
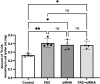
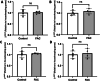
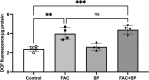
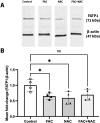
Results: FAC (500 µM, 72 h) had no impact on the expression of FABP5 at the protein and mRNA level in hCMEC/D3 cells, which was associated with a lack of effect on the uptake of 14C-DHA. FAC led to a 19.7% reduction in FATP1 protein abundance in hCMEC/D3 cells with no impact on mRNA levels, and this was associated with up to a 32.6% reduction in efflux of 14C-DHA.
Conclusions: These studies demonstrate a role of iron in down-regulating FATP1 protein abundance and function at the BBB, which may have implications on fatty acid access to the brain.
Keywords: blood-brain barrier; docosahexaenoic acid; fatty acid binding protein 5; fatty acid transport protein 1; iron.
© 2024. The Author(s).
Figures



References
-
- Corrigan FM, Horrobin DF, Skinner ER, Besson JA, Cooper MB. Abnormal content of n-6 and n-3 long-chain unsaturated fatty acids in the phosphoglycerides and cholesterol esters of parahippocampal cortex from Alzheimer’s disease patients and its relationship to acetyl CoA content. Int J Biochem Cell Biol. 1998;30(2):197–207. 10.1016/S1357-2725(97)00125-8 - DOI - PubMed
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Research Materials
Miscellaneous

