Residual Nodal Burden After Neoadjuvant Chemotherapy in cN1 Breast Cancer Patients with Positive Nodes at Targeted Axillary Dissection
- PMID: 39044106
- PMCID: PMC12335219
- DOI: 10.1245/s10434-024-15797-6
Residual Nodal Burden After Neoadjuvant Chemotherapy in cN1 Breast Cancer Patients with Positive Nodes at Targeted Axillary Dissection
Abstract
Background: Targeted axillary dissection (TAD) facilitates nodal staging in cN1 breast cancer after neoadjuvant chemotherapy (NAC). Completion axillary node dissection (cALND) remains the standard of care for TAD-positive patients. This study investigated factors associated with additional positive nodes at cALND (cALND+) and the impact on the residual cancer burden (RCB).
Methods: Retrospective review of cN1 breast cancer patients treated with NAC and TAD was conducted from July 2013 to June 2023. The review defined cN1 status by ultrasound (US) and biopsy. Patient, tumor, and treatment characteristics were evaluated. Multivariate analysis was performed to identify factors associated with cALND+, and RCB was calculated.
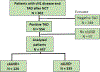
Results: Of 902 patients who underwent TAD, 554 (61.4%) were TAD-positive. 457 underwent cALND, and 124 (27%) were cALND+ (average 4.1 additional +nodes). The cALND+ patients had larger primary tumors at diagnosis (4 vs 3.5 cm; p = 0.04), more than three suspicious nodes on initial US (30% vs 13%; p ≤ 0.0001), larger residual primary tumors on pathology (median, 3 vs 2.1 cm; p = 0.0004), and more positive TAD nodes (median, 2 vs 1; p ≤ 0.0001). In the multivariate analysis, the factors associated with cALND+ were more than three suspicious nodes on initial US (odds ratio [OR], 2.9; p ≤ 0.0001), more positive TAD nodes (OR, 1.1; p ≤ 0.0001), larger clipped node metastasis (OR, 1.1; p ≤ 0.0001), and larger residual tumor on pathology (OR, 1.1; p = 0.006). Of 65 cALND+ patients with RCB class I or II, 29 (45%) had an increase in RCB based on cALND.
Conclusion: Of cN1 breast cancer patients treated with NAC who are TAD-positive, approximately 25% will have additional nodal disease on cALND. In these patients, positive cALND is associated with greater disease burden, which has potential implications for RCB status and prognosis.
Keywords: Breast cancer; Completion axillary dissection; Neoadjuvant chemotherapy; Node-positive; Targeted axillary dissection.
© 2024. Society of Surgical Oncology.
Conflict of interest statement
Disclosures:
Kelly K. Hunt reports – Medical Advisory Board – ArmadaHealth, AstraZeneca; Research funding to MD Anderson Cancer Center – Cairn Surgical, Eli Lilly & Co., Lumicell.
Benjamin D. Smith reports - research funding from Artidis, past salary support from Varian Medical Systemc, and royalty and equity interest in Oncora Medical.
Wei Yang reports royalties from Elsevier.
Alexandra M. Moore reports – AstraZeneca paid speaker at SSO Annual Meeting 2024.
Figures
References
-
- Fisher B, Brown A, Mamounas E, et al. Effect of preoperative chemotherapy on local-regional disease in women with operable breast cancer: findings from National Surgical Adjuvant Breast and Bowel Project B-18. J Clin Oncol. 1997;15(7):2483–2493. - PubMed
-
- Bear HD, Anderson S, Smith RE, et al. Sequential preoperative or postoperative docetaxel added to preoperative doxorubicin plus cyclophosphamide for operable breast cancer:National Surgical Adjuvant Breast and Bowel Project Protocol B-27. J Clin Oncol. 2006;24(13):2019–2027. - PubMed
-
- Masuda N, Lee SJ, Ohtani S, et al. Adjuvant Capecitabine for Breast Cancer after Preoperative Chemotherapy. N Engl J Med. 2017;376(22):2147–2159. - PubMed
-
- von Minckwitz G, Huang CS, Mano MS, et al. Trastuzumab Emtansine for Residual Invasive HER2-Positive Breast Cancer. N Engl J Med. 2019;380(7):617–628. - PubMed
MeSH terms
Grants and funding
LinkOut - more resources
Full Text Sources
Medical
Miscellaneous