Mapping the evidence about the natural history of acute infections commonly seen in primary care and managed with antibiotics: a scoping review
- PMID: 39044144
- PMCID: PMC11264388
- DOI: 10.1186/s12879-024-09526-3
Mapping the evidence about the natural history of acute infections commonly seen in primary care and managed with antibiotics: a scoping review
Abstract
Background: Knowing the natural history of acute infections in primary care, defined as the course of a disease over time in the absence of specific therapy or treatment, can inform clinicians' and patients' expectations about illness recovery, but this evidence is fragmented across the literature. This scoping review aimed to map existing research and research gaps relevant to the natural history of acute infections.
Methods: We searched MEDLINE, Embase and CENTRAL using a 2-phase hierarchical search approach. In Phase A, we focused on identifying systematic reviews synthesising natural history data for eligible infections (acute respiratory, urinary, and skin and soft tissue) and systematic reviews of treatment effectiveness (of RCTs with placebo or no treatment arm, or cohort studies). For infections without existing reviews, in Phase B, we searched for primary studies (placebo-controlled RCTs or cohort studies). Two reviewers independently screened and extracted the data (study characteristics, outcome data - e.g., symptom duration, proportion with resolution at various time points).
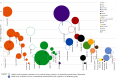
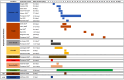
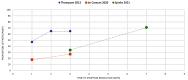
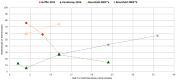
Results: We identified 40 systematic reviews, reporting on 45 infections, most commonly (90%) respiratory tract infections. Six (15%) of these aimed to synthesise natural history information. Most reviews reported the proportion of participants with symptom resolution at various time point/s, with 58% providing data on mean symptom duration. Recovery data show the spontaneous resolution of some infections in some people. We found no eligible studies for cellulitis, ecthyma, carbuncle, and erysipelas.
Conclusions: Our review has shown that natural history evidence exists for many common acute infections. It can be utilised by clinicians in implementing patient-centred antibiotic stewardship strategies in primary care. Future research should focus on generating natural history evidence for skin and soft tissue infections and urinary tract infections.
Keywords: Acute infections; Antibiotic stewardship; Natural history; Primary care; Respiratory tract infections.
© 2024. The Author(s).
Conflict of interest statement
The authors declare no competing interests.
Figures







References
-
- World Health Organisation. Global action plan on antimicrobial resistance. 2015. 2015 [cited 2023 28 Aug]. https://www.who.int/publications/i/item/9789241509763.
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Medical

