Astrocytic autophagy plasticity modulates Aβ clearance and cognitive function in Alzheimer's disease
- PMID: 39044253
- PMCID: PMC11267931
- DOI: 10.1186/s13024-024-00740-w
Astrocytic autophagy plasticity modulates Aβ clearance and cognitive function in Alzheimer's disease
Abstract
Background: Astrocytes, one of the most resilient cells in the brain, transform into reactive astrocytes in response to toxic proteins such as amyloid beta (Aβ) in Alzheimer's disease (AD). However, reactive astrocyte-mediated non-cell autonomous neuropathological mechanism is not fully understood yet. We aimed our study to find out whether Aβ-induced proteotoxic stress affects the expression of autophagy genes and the modulation of autophagic flux in astrocytes, and if yes, how Aβ-induced autophagy-associated genes are involved Aβ clearance in astrocytes of animal model of AD.
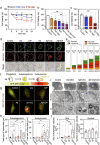
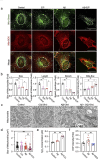
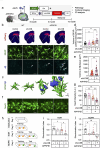
Methods: Whole RNA sequencing (RNA-seq) was performed to detect gene expression patterns in Aβ-treated human astrocytes in a time-dependent manner. To verify the role of astrocytic autophagy in an AD mouse model, we developed AAVs expressing shRNAs for MAP1LC3B/LC3B (LC3B) and Sequestosome1 (SQSTM1) based on AAV-R-CREon vector, which is a Cre recombinase-dependent gene-silencing system. Also, the effect of astrocyte-specific overexpression of LC3B on the neuropathology in AD (APP/PS1) mice was determined. Neuropathological alterations of AD mice with astrocytic autophagy dysfunction were observed by confocal microscopy and transmission electron microscope (TEM). Behavioral changes of mice were examined through novel object recognition test (NOR) and novel object place recognition test (NOPR).
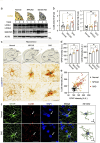
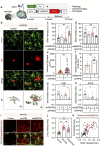
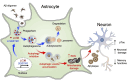
Results: Here, we show that astrocytes, unlike neurons, undergo plastic changes in autophagic processes to remove Aβ. Aβ transiently induces expression of LC3B gene and turns on a prolonged transcription of SQSTM1 gene. The Aβ-induced astrocytic autophagy accelerates urea cycle and putrescine degradation pathway. Pharmacological inhibition of autophagy exacerbates mitochondrial dysfunction and oxidative stress in astrocytes. Astrocyte-specific knockdown of LC3B and SQSTM1 significantly increases Aβ plaque formation and GFAP-positive astrocytes in APP/PS1 mice, along with a significant reduction of neuronal marker and cognitive function. In contrast, astrocyte-specific overexpression of LC3B reduced Aβ aggregates in the brain of APP/PS1 mice. An increase of LC3B and SQSTM1 protein is found in astrocytes of the hippocampus in AD patients.
Conclusions: Taken together, our data indicates that Aβ-induced astrocytic autophagic plasticity is an important cellular event to modulate Aβ clearance and maintain cognitive function in AD mice.
Keywords: Alzheimer’s disease; Amyloid beta (Aβ); Astrocytes; Autophagy; Aβ clearance; Mitochondria.
© 2024. The Author(s).
Conflict of interest statement
The authors declare that there is no competing financial interests.
Figures



References
-
- Carter SF, Scholl M, Almkvist O, Wall A, Engler H, Langstrom B, Nordberg A. Evidence for astrocytosis in prodromal Alzheimer disease provided by 11 C-deuterium-L-deprenyl: a multitracer PET paradigm combining 11 C-Pittsburgh compound B and 18F-FDG. J Nucl Med. 2012;53:37–46. 10.2967/jnumed.110.087031 - DOI - PubMed
-
- Chun H, Lee CJ. Reactive astrocytes in Alzheimer’s disease: A double-edged sword. Neurosci Res 2017. - PubMed
-
- Jiwaji Z, Tiwari SS, Aviles-Reyes RX, Hooley M, Hampton D, Torvell M, Johnson DA, McQueen J, Baxter P, Sabari-Sankar K, et al. Reactive astrocytes acquire neuroprotective as well as deleterious signatures in response to Tau and ass pathology. Nat Commun. 2022;13:135. 10.1038/s41467-021-27702-w - DOI - PMC - PubMed
MeSH terms
Substances
Grants and funding
- 2E33411/Korea Institute of Science and Technology
- NRF-2022R1A6A3A01086375/National Research Foundation of Korea
- 2E32901/Korea Institute of Science and Technology
- 2024-22-0131/Yonsei University
- HU23C0217/National Research Foundation (NRF)
- NRF-2020M3E5D9079742, NRF-2022R1A2C3013138/National Research Foundation (NRF)
- IBS-R001-D2/Institute for Basic Science (IBS)
- R01NS109537/National Institute of Health (NIH)
- R01 NS109537/NS/NINDS NIH HHS/United States
- NRF-2021R1C1C2095827/National Research Foundation of Korea (NRF)
- 2E32901 and 2E32922/Korea Institute of Science and Technology
LinkOut - more resources
Full Text Sources
Medical
Molecular Biology Databases
Miscellaneous

