Exposure-response modeling for nausea incidence for cotadutide using a Markov modeling approach
- PMID: 39044369
- PMCID: PMC11533102
- DOI: 10.1002/psp4.13194
Exposure-response modeling for nausea incidence for cotadutide using a Markov modeling approach
Abstract
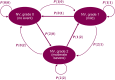
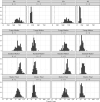
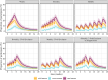
Cotadutide is a dual glucagon-like peptide-1 (GLP-1)/glucagon receptor agonist. Gastrointestinal adverse effects are known to be associated with GLP-1 receptor agonism and can be mitigated through tolerance development via a gradual up-titration. This analysis aimed to characterize the relationship between exposure and nausea incidence and to optimize titration schemes. The model was developed with pooled data from cotadutide-administrated studies. Three different modeling approaches, proportional odds (PO), discrete-time Markov, and two-stage discrete-time Markov models, were employed to characterize the exposure-nausea relationship. The severity of nausea was modeled as different states (non-nausea, mild, and moderate/severe). The most appropriate model was selected to perform the covariate analysis, and the final covariate model was used to simulate the nausea event rates for various titration scenarios. The two Markov models demonstrated comparable performance and were better than the PO model. The covariate analysis was conducted with the standard Markov model for operational simplification and identified disease indications (NASH, obesity) and sex as covariates on Markov parameters. The simulations indicated that the biweekly titration with twofold dose escalation is superior to other titration schemes with a relatively low predicted nausea event rate at 600 μg (25%) and a shorter titration interval (8 weeks) to reach the therapeutic dose. The model can be utilized to optimize starting dose and titration schemes for other therapeutics in clinical trials to achieve an optimal risk-benefit balance and reach the therapeutic dose with minimal titration steps.
© 2024 The Author(s). CPT: Pharmacometrics & Systems Pharmacology published by Wiley Periodicals LLC on behalf of American Society for Clinical Pharmacology and Therapeutics.
Conflict of interest statement
H.Y., S.U., J.C., D.R., L.H., A.F., V.P., B.H., and A.A.K. are employees of AstraZeneca and own AstraZeneca stocks or stock options. L.Z. declared no competing interests for this work.
Figures

Similar articles
-
Characterisation of cotadutide's dual GLP-1/glucagon receptor agonistic effects on glycaemic control using an in vivo human glucose regulation quantitative systems pharmacology model.Br J Pharmacol. 2024 Jun;181(12):1874-1885. doi: 10.1111/bph.16336. Epub 2024 Feb 25. Br J Pharmacol. 2024. PMID: 38403793 Clinical Trial.
-
Population Pharmacokinetic Modeling of Cotadutide: A Dual Agonist Peptide of Glucagon-Like Peptide and Glucagon Receptors Administered to Participants with Type II Diabetes Mellitus, Chronic Kidney Disease, Obesity and Non-Alcoholic Steatohepatitis.Clin Pharmacokinet. 2024 Feb;63(2):255-267. doi: 10.1007/s40262-023-01337-0. Epub 2024 Jan 18. Clin Pharmacokinet. 2024. PMID: 38236561
-
Clinical factors associated with the occurrence of nausea and vomiting in type 2 diabetes patients treated with glucagon-like peptide-1 receptor agonists.J Diabetes Investig. 2019 Mar;10(2):408-417. doi: 10.1111/jdi.12900. Epub 2018 Aug 22. J Diabetes Investig. 2019. PMID: 30033675 Free PMC article.
-
Occurrence of nausea, vomiting and diarrhoea reported as adverse events in clinical trials studying glucagon-like peptide-1 receptor agonists: A systematic analysis of published clinical trials.Diabetes Obes Metab. 2017 Mar;19(3):336-347. doi: 10.1111/dom.12824. Epub 2016 Dec 19. Diabetes Obes Metab. 2017. PMID: 27860132 Review.
-
Efficacy and safety of high-dose glucagon-like peptide-1, glucagon-like peptide-1/glucose-dependent insulinotropic peptide, and glucagon-like peptide-1/glucagon receptor agonists in type 2 diabetes.Diabetes Obes Metab. 2022 May;24(5):788-805. doi: 10.1111/dom.14640. Epub 2022 Jan 21. Diabetes Obes Metab. 2022. PMID: 34984793 Review.
Cited by
-
GLP-1 and Its Analogs: Does Sex Matter?Endocrinology. 2025 Jan 6;166(2):bqae165. doi: 10.1210/endocr/bqae165. Endocrinology. 2025. PMID: 39715341 Free PMC article. Review.
References
-
- Drucker DJ, Nauck MA. The incretin system: glucagon‐like peptide‐1 receptor agonists and dipeptidyl peptidase‐4 inhibitors in type 2 diabetes. Lancet. 2006;368(9548):1696‐1705. - PubMed
-
- Bettge K, Kahle M, Abd El Aziz MS, Meier JJ, Nauck MA. Occurrence of nausea, vomiting and diarrhoea reported as adverse events in clinical trials studying glucagon‐like peptide‐1 receptor agonists: a systematic analysis of published clinical trials. Diabetes Obes Metab. 2017;19(3):336‐347. - PubMed
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical

