Amyloid and Tau Prediction of Cognitive and Functional Decline in Unimpaired Older Individuals: Longitudinal Data from the A4 and LEARN Studies
- PMID: 39044488
- PMCID: PMC11266444
- DOI: 10.14283/jpad.2024.122
Amyloid and Tau Prediction of Cognitive and Functional Decline in Unimpaired Older Individuals: Longitudinal Data from the A4 and LEARN Studies
Abstract
Background: Converging evidence suggests that markers of Alzheimer's disease (AD) pathology in cognitively unimpaired older individuals are associated with high risk of cognitive decline and progression to functional impairment. The Anti-Amyloid Treatment in Asymptomatic Alzheimer's disease (A4) and Longitudinal Evaluation of Amyloid and Neurodegeneration Risk (LEARN) Studies enrolled a large cohort of cognitively normal older individuals across a range of baseline amyloid PET levels. Recent advances in AD blood-based biomarkers further enable the comparison of baseline markers in the prediction of longitudinal clinical outcomes.
Objectives: We sought to evaluate whether biomarker indicators of higher levels of AD pathology at baseline predicted greater cognitive and functional decline, and to compare the relative predictive power of amyloid PET imaging, tau PET imaging, and a plasma P-tau217 assay.
Design: All participants underwent baseline amyloid PET scan, plasma P-tau217; longitudinal cognitive testing with the Primary Alzheimer Cognitive Composite (PACC) every 6 months; and annual functional assessments with the clinical dementia rating (CDR), cognitive functional index (CFI), and activities of daily living (ADL) scales. Baseline tau PET scans were obtained in a subset of participants. Participants with elevated amyloid (Aβ+) on screening PET who met inclusion/exclusion criteria were randomized to receive placebo or solanezumab in a double-blind phase of the A4 Study over 240+ weeks. Participants who did not have elevated amyloid (Aβ-) but were otherwise eligible for the A4 Study were referred to the companion observational LEARN Study with the same outcome assessments over 240+ weeks.
Setting: The A4 and LEARN Studies were conducted at 67 clinical trial sites in the United States, Canada, Japan and Australia.
Participants: Older participants (ages 65-85) who were cognitively unimpaired at baseline (CDR-GS=0, MMSE 25-30 with educational adjustment, and Logical Memory scores within the normal range LMIIa 6-18) were eligible to continue in screening. Aβ+ participants were randomized to either placebo (n=583) or solanezumab (n=564) in the A4 Study. A subset of Aβ+ underwent tau PET imaging in A4 (n=350). Aβ- were enrolled into the LEARN Study (n=553).
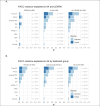
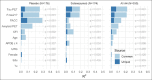
Measurements: Baseline 18-F Florbetapir amyloid PET, 18-F Flortaucipir tau PET in a subset and plasma P-tau217 with an electrochemiluminescence (ECL) immunoassay were evaluated as predictors of cognitive (PACC), and functional (CDR, CFI and ADL) change. Models were evaluated to explore the impact of baseline tertiles of amyloid PET and tertiles of plasma P-tau217 on cognitive and functional outcomes in the A4 Study compared to LEARN. Multivariable models were used to evaluate the unique and common variance explained in longitudinal outcomes based on baseline predictors, including effects for age, gender, education, race/ethnic group, APOEε4 carrier status, baseline PACC performance and treatment assignment in A4 participants (solanezumab vs placebo).
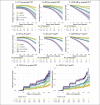
Results: Higher baseline amyloid PET CL and P-tau217 levels were associated with faster rates of PACC decline, and increased likelihood of progression to functional impairment (CDR 0.5 or higher on two consecutive measurements), both across LEARN Aβ- and A4 Aβ+ (solanezumab and placebo arms). In analyses considering all baseline predictor variables, P-tau217 was the strongest predictor of PACC decline. Among participants in the highest tertiles of amyloid PET or P-tau217, >50% progressed to CDR 0.5 or greater. In the tau PET substudy, neocortical tau was the strongest predictor of PACC decline, but plasma P-tau217 contributed additional independent predictive variance in commonality variance models.
Conclusions: In a large cohort of cognitively unimpaired individuals enrolled in a Phase 3 clinical trial and companion observational study, these findings confirm that higher baseline levels of amyloid and tau markers are associated with increased rates of cognitive decline and progression to functional impairment. Interestingly, plasma P-tau217 was the best predictor of decline in the overall sample, superior to baseline amyloid PET. Neocortical tau was the strongest predictor of cognitive decline in the subgroup with tau PET, suggesting that tau deposition is most closely linked to clinical decline. These findings indicate that biomarkers of AD pathology are useful to predict decline in an older asymptomatic population and may prove valuable in the selection of individuals for disease-modifying treatments.
Keywords: Amyloid; biomarkers; cognitive decline; imaging; tau.
Conflict of interest statement
RAS reports grant support from the National Institutes on Aging, National Institutes of Health, Alzheimer’s Association, GHR Foundation, and Gates Ventures. She has received trial research funding from Eisai and Eli Lilly for public-private partnership trials. She reported serving as a consultant for AbbVie, AC Immune, Alector, Biohaven, Bristol-Myers-Squibb, Ionis, Janssen, Genentech, Merck, Prothena, Roche, and Vaxxinity. MCD has received research funding from the National Institutes of Health, Janssen, Eli Lilly, and Eisai, reports consulting fees from Roche and his spouse is a full-time employee of Janssen. KAJ has received research funding from National Institutes on Aging, National Institutes of Health, Alzheimer’s Association, and the GHR Foundation. He has served as a consultant for Merck, Novartis, Janssen, and Prothena. RAR has research support from the National Institute on Aging, the Alzheimer’s Association and is a consultant for Amydis Inc, Bioivt, Lexeo, Keystone Bio, Allyx, DiamiR, Ionis and PrecisionMed. CJ has received research support from the National Institutes of Health (NIH), the Alzheimer’s Association, American Heart Association, Eli Lilly and Eisai. DMR received salary and research support from the National Institutes of Health and has received payment or honoraria from USC Institute on Methods and Protocols for Advancement of Clinical Trials in ADRD (IMPACT AD) course and External Advisory Boards from the University of California-Davis, Washington University, Boston University and Northwestern. She has also received travel support to ACTC meetings, to the University of California Advisory Board Meeting and the Washington University Advisory Board Meeting. JG has received research support from the National Institutes of Health (NIH), the Alzheimer’s Association, BrightFocus Foundation, Eli Lilly, Biogen, Genentech, and Eisai. He has received personal fees for providing consulting to SiteRx and editorial service to Alzheimer’s and Dementia. JLH has received research support from the National Institutes of Health, the Alzheimer’s Association, Eli Lilly, and Eisai and has received honoraria from USC Institute on Methods and Protocols for Advancement of Clinical Trials in ADRD (IMPACT AD) RR has received research support from the National Institutes of Health (NIH), the Alzheimer’s Association, American Heart Association, Eli Lilly and Eisai. AL has received research support from the National Institutes of Health (NIH), the Alzheimer’s Association, American Heart Association, Eli Lilly and Eisai. OL has received research support from the National Institutes of Health (NIH), the Alzheimer’s Association, American Heart Association, Eli Lilly and Eisai. GJM has received research support from the National Institutes of Health (NIH), the Alzheimer’s Association, American Heart Association, Gates Ventures, Eli Lilly, and Eisai. RY is an employee and minor shareholder of Eli Lilly and Company. KCH is an employee and minor shareholder of Eli Lilly and Company. JS is an employee and minor shareholder of Eli Lilly and Company. PSA has received grants or contracts from the National Institutes of Health (NIH), Alzheimer’s Association, Foundation for NIH (FNIH), Lilly, Janssen and Eisai and consulting fees from Merck, Biogen, AbbVie, Roche, and Immunobrain Checkpoint.
Figures

References
-
- Ossenkoppele R, Smith R, Mattsson-Carlgren N, Groot C, Leuzy A, Strandberg O, et al. Accuracy of Tau Positron Emission Tomography as a Prognostic Marker in Preclinical and Prodromal Alzheimer Disease: A Head-to-Head Comparison Against Amyloid Positron Emission Tomography and Magnetic Resonance Imaging. JAMA Neurol. 2021;78(8):961–971. 10.1001/jamaneurol.2021.1858 PubMed PMID: 34180956; PMCID 8240013. - DOI - PMC - PubMed
-
- Jack CR, Jr., Wiste HJ, Algeciras-Schimnich A, Weigand SD, Figdore DJ, Lowe VJ, et al. Comparison of plasma biomarkers and amyloid PET for predicting memory decline in cognitively unimpaired individuals. Alzheimers Dement. 2024;20(3):2143–2154. 10.1002/alz.13651 PubMed PMID: 38265198; PMCID 10984437. - DOI - PMC - PubMed
-
- Sperling RA, Aisen PS, Beckett LA, Bennett DA, Craft S, Fagan AM, et al. Toward defining the preclinical stages of Alzheimer's disease: Recommendations from the National Institute on Aging-Alzheimer's Association workgroups on diagnostic guidelines for Alzheimer's disease. Alzheimers Dement. 2011;7(3):280–292. 10.1016/j.jalz.2011.03.003 PubMed PMID: 21514248; S1552-5260(11)00099-9 [pii] - DOI - PMC - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical
Miscellaneous

