Longitudinal Phospho-tau217 Predicts Amyloid Positron Emission Tomography in Asymptomatic Alzheimer's Disease
- PMID: 39044490
- PMCID: PMC11266279
- DOI: 10.14283/jpad.2024.134
Longitudinal Phospho-tau217 Predicts Amyloid Positron Emission Tomography in Asymptomatic Alzheimer's Disease
Abstract
Background: Blood-based AD biomarkers such as plasma P-tau217 are increasingly used in clinical trials as a screening tool.
Objectives: To assess the utility of an electrochemiluminescence (ECL) immunoassay in predicting brain amyloid PET status in cognitively unimpaired individuals.
Setting: Plasma samples collected at baseline, week 12, and week 240 or endpoint originated from the Anti-Amyloid Treatment in Asymptomatic Alzheimer's Disease (A4) trial and the companion Longitudinal Evaluation of Amyloid Risk and Neurodegeneration (LEARN) study.
Participants: Both A4 and LEARN enrolled eligible cognitively unimpaired persons 65 to 85 years. Individuals with elevated brain amyloid PET levels were eligible for the A4 Study, while those without elevated brain amyloid PET levels were eligible for the LEARN Study.
Intervention: Participants in the A4 Study received intravenous solanezumab (up to 1600 mg) or placebo every 4 weeks. The LEARN Study is an observational study without intervention.
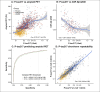
Measurements: Plasma P-tau217 concentration levels from A4 Study participants were measured using an ECL immunoassay. Receiver Operating Characteristic (ROC) curve analysis was performed for each biomarker against amyloid positivity, defined by ≥22 CL and ≥ 33 CL.
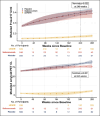
Results: Receiver operating characteristic curve (ROC) analysis indicates high diagnostic value of P-tau217 in individuals with amyloid PET ≥ 20 (Area under the ROC (AUROC): 0.87) and ≥ 33 CL (AUROC: 0.89). Repeated testing with the placebo group taken 12 weeks apart (range: 68 to 143 days) and the LEARN participants taken between 1.4 and 1.75 years resulted in a strong positive correlation (Corr. 0.91 (0.90 to 0.92)).
Conclusion: An ECL immunoassay testing plasma P-tau217 accurately predicts amyloid PET positivity in cognitively unimpaired individuals. Our future analyses aim to determine if use of this assay may reduce the screening burden of preclinical individuals into anti-amyloid clinical trials.
Keywords: Alzheimer’s disease; PET; immunoassay; longitudinal amyloid; p-tau.
Conflict of interest statement
RAR: has research support from the National Institute on Aging, the Alzheimer’s Association and is a consultant for Amydis Inc, Bioivt, Lexeo, Keystone Bio, Allyx, DiamiR, Ionis and PrecisionMed. MD: Nothing to report. OL: Nothing to report. SAL: Nothing to report. RR has received research support from the National Institutes of Health (NIH), the Alzheimer’s Association, American Heart Association, Eli Lilly and Eisai. GJM has received research support from the National Institutes of Health (NIH), the Alzheimer’s Association, American Heart Association, Gates Ventures, Eli Lilly, and Eisai. KAJ has received research funding from National Institutes on Aging, National Institutes of Health, Alzheimer’s Association, and the GHR Foundation. He has served as a consultant for Merkc, Novartis, Janssen, and Prothena. DMR received salary and research support from the National Institutes of Health and has received payment or honoraria from USC Institute on Methods and Protocols for Advancement of Clinical Trials in ADRD (IMPACT AD) course and External Advisory Boards from the University of California-Davis, Washington University, Boston University and Northwestern. She has also received travel support to ACTC meetings, to the University of California Advisory Board Meeting and the Washington University Advisory Board Meeting. RY is an employee and minor shareholder of Eli Lilly and Company. KCH is an employee and minor shareholder of Eli Lilly and Company. JRS is an employee and minor shareholder of Eli Lilly and Company. PSA has received grants or contracts from the National Institutes of Health (NIH), Alzheimer’s Association, Foundation for NIH (FNIH), Lilly, Janssen and Eisai and consulting fees from Merck, Biogen, AbbVie, Roche, and Immunobrain Checkpoint. RAS reports grant support from the National Institutes on Aging, National Institutes of Health, Alzheimer’s Association, GHR Foundation, and Gates Ventures. She has received trial research funding from Eisai and Eli Lilly for public-private partnership trials. She reported serving as a consultant for AbbVie, AC Immune, Alector, Biohaven, Bristol-Myers-Squibb, Ionis, Janssen, Genentech, Merck, Prothena, Roche, and Vaxxinity.
Figures

References
-
- Rajan KB, Weuve J, Barnes LL, McAninch EA, Wilson RS, Evans DA. Population estimate of people with clinical Alzheimer's disease and mild cognitive impairment in the United States (2020–2060) Alzheimer' & Dementia. 2021;17(12):1966–1975. 10.1002/alz.12362 - DOI - PMC - PubMed
-
- Jack CR, Bennett DA, Blennow K, Carrillo MC, Dunn B, Haeberlein SB, et al. NIA-AA Research Framework: Toward a biological definition of Alzheimer's disease. Alzheimer's & Dementia. 2018;14(4):535–562. 10.1016/j.jalz.2018.02.018 - DOI - PMC - PubMed
-
- Dubois B, Feldman HH, Jacova C, Dekosky ST, Barberger-Gateau P, Cummings J, et al. Research criteria for the diagnosis of Alzheimer's disease: revising the NINCDS-ADRDA criteria. Lancet Neurol. 2007;6(8):734–746. 10.1016/S1474-4422(07)70178-3 PubMed PMID: 17616482. - DOI - PubMed
-
- Sperling RA, Jack CR, Aisen PS. Testing the Right Target and Right Drug at the Right Stage. Science Translational Medicine. 2011;3(111):111c. 10.1126/scitranslmed.3002609 33–cm33. - DOI - PMC - PubMed
-
- Donohue MC, Sperling RA, Petersen R, Sun CK, Weiner MW, Aisen PS, Initiative AsDN Association Between Elevated Brain Amyloid and Subsequent Cognitive Decline Among Cognitively Normal Persons. JAMA. 2017;317(22):2305–2316. 10.1001/jama.2017.6669 PubMed PMID: 28609533; PMCID 5736301. - DOI - PMC - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical

