Biochemical hallmarks-targeting antineoplastic nanotherapeutics
- PMID: 39044728
- PMCID: PMC11263727
- DOI: 10.1016/j.bioactmat.2024.05.042
Biochemical hallmarks-targeting antineoplastic nanotherapeutics
Abstract
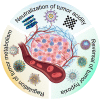
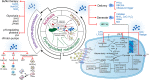
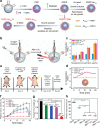
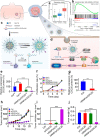
Tumor microenvironments (TMEs) have received increasing attention in recent years as they play pivotal roles in tumorigenesis, progression, metastases, and resistance to the traditional modalities of cancer therapy like chemotherapy. With the rapid development of nanotechnology, effective antineoplastic nanotherapeutics targeting the aberrant hallmarks of TMEs have been proposed. The appropriate design and fabrication endow nanomedicines with the abilities for active targeting, TMEs-responsiveness, and optimization of physicochemical properties of tumors, thereby overcoming transport barriers and significantly improving antineoplastic therapeutic benefits. This review begins with the origins and characteristics of TMEs and discusses the latest strategies for modulating the TMEs by focusing on the regulation of biochemical microenvironments, such as tumor acidosis, hypoxia, and dysregulated metabolism. Finally, this review summarizes the challenges in the development of smart anti-cancer nanotherapeutics for TME modulation and examines the promising strategies for combination therapies with traditional treatments for further clinical translation.
Keywords: Biochemical hallmark; Cancer therapy; Controlled drug delivery; Nanoparticle; Tumor microenvironment.
© 2024 The Authors.
Conflict of interest statement
The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
Figures



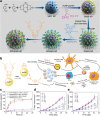
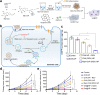
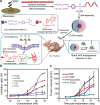
References
-
- Lozano R., Naghavi M., Foreman K., Lim S., Shibuya K., Aboyans V., Abraham J., Adair T., Aggarwal R., Ahn S.Y., Alvarado M., Anderson H.R., Anderson L.M., Andrews K.G., Atkinson C., Baddour L.M., Barker-Collo S., Bartels D.H., Bell M.L., Benjamin E.J., Bennett D., Bhalla K., Bikbov B., Bin A.A., Birbeck G., Blyth F., Bolliger I., Boufous S., Bucello C., Burch M., Burney P., Carapetis J., Chen H., Chou D., Chugh S.S., Coffeng L.E., Colan S.D., Colquhoun S., Colson K.E., Condon J., Connor M.D., Cooper L.T., Corriere M., Cortinovis M., de Vaccaro K.C., Couser W., Cowie B.C., Criqui M.H., Cross M., Dabhadkar K.C., Dahodwala N., De Leo D., Degenhardt L., Delossantos A., Denenberg J., Des Jarlais D.C., Dharmaratne S.D., Dorsey E.R., Driscoll T., Duber H., Ebel B., Erwin P.J., Espindola P., Ezzati M., Feigin V., Flaxman A.D., Forouzanfar M.H., Fowkes F.G., Franklin R., Fransen M., Freeman M.K., Gabriel S.E., Gakidou E., Gaspari F., Gillum R.F., Gonzalez-Medina D., Halasa Y.A., Haring D., Harrison J.E., Havmoeller R., Hay R.J., Hoen B., Hotez P.J., Hoy D., Jacobsen K.H., James S.L., Jasrasaria R., Jayaraman S., Johns N., Karthikeyan G., Kassebaum N., Keren A., Khoo J.P., Knowlton L.M., Kobusingye O., Koranteng A., Krishnamurthi R., Lipnick M., Lipshultz S.E., Ohno S.L., Mabweijano J., MacIntyre M.F., Mallinger L., March L., Marks G.B., Marks R., Matsumori A., Matzopoulos R., Mayosi B.M., McAnulty J.H., McDermott M.M., McGrath J., Mensah G.A., Merriman T.R., Michaud C., Miller M., Miller T.R., Mock C., Mocumbi A.O., Mokdad A.A., Moran A., Mulholland K., Nair M.N., Naldi L., Narayan K.M., Nasseri K., Norman P., O’Donnell M., Omer S.B., Ortblad K., Osborne R., Ozgediz D., Pahari B., Pandian J.D., Rivero A.P., Padilla R.P., Perez-Ruiz F., Perico N., Phillips D., Pierce K., Pope C.A.R., Porrini E., Pourmalek F., Raju M., Ranganathan D., Rehm J.T., Rein D.B., Remuzzi G., Rivara F.P., Roberts T., De Leon F.R., Rosenfeld L.C., Rushton L., Sacco R.L., Salomon J.A., Sampson U., Sanman E., Schwebel D.C., Segui-Gomez M., Shepard D.S., Singh D., Singleton J., Sliwa K., Smith E., Steer A., Taylor J.A., Thomas B., Tleyjeh I.M., Towbin J.A., Truelsen T., Undurraga E.A., Venketasubramanian N., Vijayakumar L., Vos T., Wagner G.R., Wang M., Wang W., Watt K., Weinstock M.A., Weintraub R., Wilkinson J.D., Woolf A.D., Wulf S., Yeh P.H., Yip P., Zabetian A., Zheng Z.J., Lopez A.D., Murray C.J., AlMazroa M.A., Memish Z.A. Global and regional mortality from 235 causes of death for 20 age groups in 1990 and 2010: A systematic analysis for the Global Burden of Disease Study 2010. Lancet. 2012;380(9859):2095–2128. - PMC - PubMed
-
- Luo C., Sun J., Sun B., He Z. Prodrug-based nanoparticulate drug delivery strategies for cancer therapy. Trends Pharmacol. Sci. 2014;35(11):556–566. - PubMed
-
- Gao S., Yang X., Xu J., Qiu N., Zhai G. Nanotechnology for boosting cancer immunotherapy and remodeling tumor microenvironment: The horizons in cancer treatment. ACS Nano. 2021;15(8):12567–12603. - PubMed
-
- Yang K., Yang Z., Yu G., Nie Z., Wang R., Chen X. Polyprodrug nanomedicines: An emerging paradigm for cancer therapy. Adv. Mater. 2022;34(6) - PubMed
Publication types
LinkOut - more resources
Full Text Sources

